CME
Decera Clinical Education Independent Conference Highlights of the San Antonio Breast Cancer Symposium 2025: Advanced and Metastatic Breast Cancer
Physicians: Maximum of 0.50 AMA PRA Category 1 Credit™
Released: February 20, 2026
Expiration: August 19, 2026
Activity
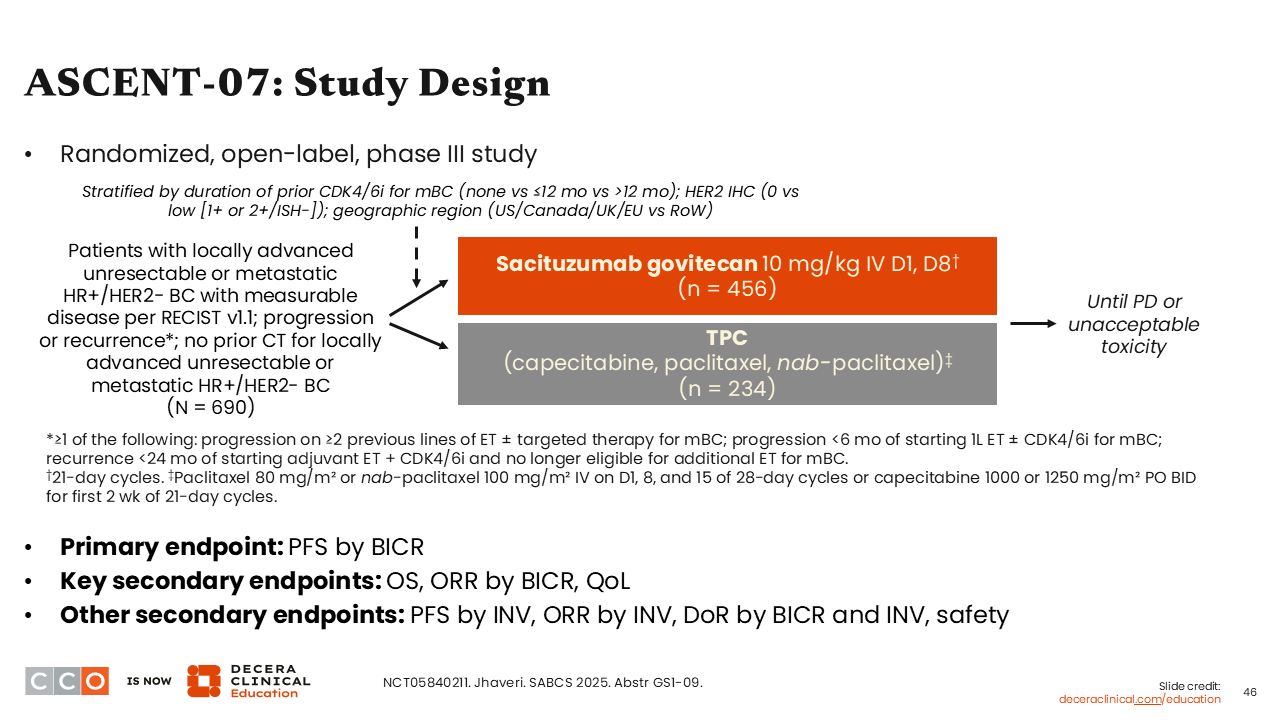
ASCENT-07: Study Design
Sara M. Tolaney, MD, MPH:
Another trial that was highly anticipated at SABCS 2025 was the phase III ASCENT-07 trial exploring sacituzumab govitecan vs treatment of physician choice (TPC; either capecitabine, paclitaxel, or nab-paclitaxel) in patients with locally advanced or metastatic HR-positive/HER2-negative breast cancer with disease progression or recurrence on 2 or more lines of ET with or without targeted therapy for mBC, or progression in less than 6 months from starting first-line ET with or without a CDK4/6 inhibitor for MBC, or recurrence <24 months of starting adjuvant ET plus CDK4/6 inhibitor and no longer eligible for additional ET for mBC (N = 690). Previous treatment with chemotherapy was not allowed in the advanced or metastatic setting. The primary endpoint was PFS.33
Sacituzumab govitecan is currently indicated for the treatment of HR-positive/HER2-negative, unresectable locally advanced or MBC after prior ET and at least 2 lines of systemic therapy in the metastatic setting based on the phase III TROPiCS-02 trial. In that trial, sacituzumab govitecan improved median PFS (5.5 vs 4.0 months; P = .0003) and median OS (14.4 vs 11.2 months; P = .020) for that patient population.34
Turning our attention back to the ASCENT-07 trial, and because of the encouraging results from TROPICS-02, investigators explored the possibility of moving sacituzumab govitecan earlier in the treatment setting for advanced and metastatic disease.
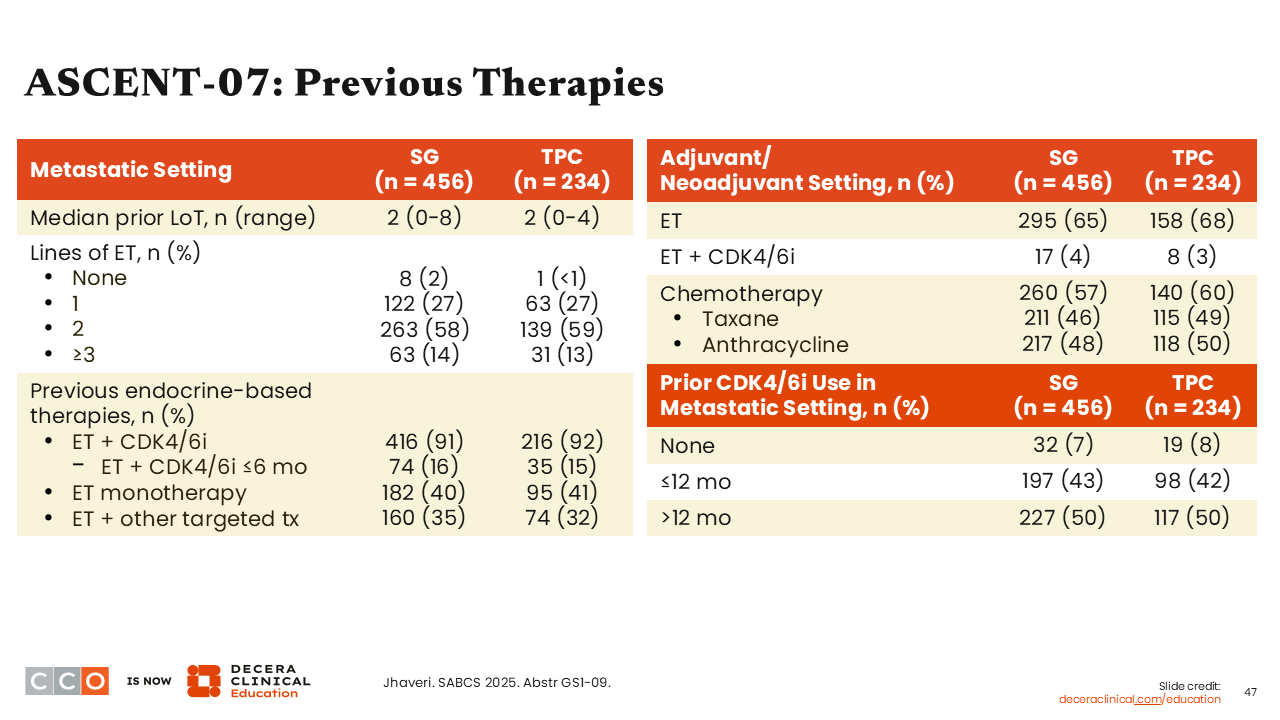
ASCENT-07: Previous Therapies
Sara M. Tolaney, MD, MPH:
Most patients enrolled in the ASCENT-07 trial had prior treatment with ET with a CDK4/6 inhibitor (91% in sacituzumab govitecan arm and 92% in the TPC arm).33 This is consistent with current practice patterns. Overall the treatment arms were balanced regarding previous treatment in the metastatic and adjuvant/neoadjuvant settings.
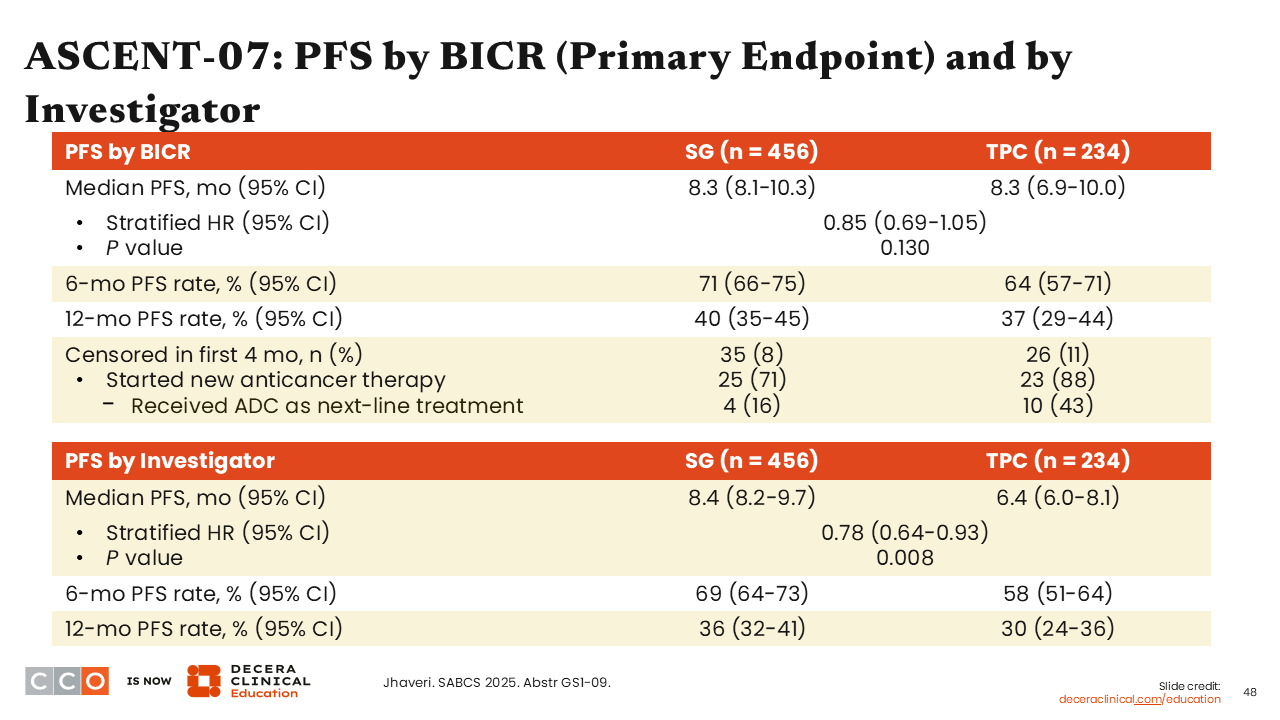
ASCENT-07: PFS by BICR (Primary Endpoint) and by Investigator
Sara M. Tolaney, MD, MPH:
The study did not meet the primary endpoint of improving PFS by BICR. There was no difference in median PFS between sacituzumab govitecan and TPC in patients who had not received prior treatment with chemotherapy for advanced/metastatic disease (8.3 months vs 8.3 months; stratified HR: 0.85; 95% CI: 0.69-1.05; P value = .130).33
Furthermore, there was a discrepancy between PFS assessed by BICR vs investigator-assessed PFS. Investigator-assessed median PFS was 8.4 months in the sacituzumab govitecan arm vs 6.4 months in the chemotherapy arm (stratified HR: 0.78; 95% CI: 0.64-0.93; P = .008).33
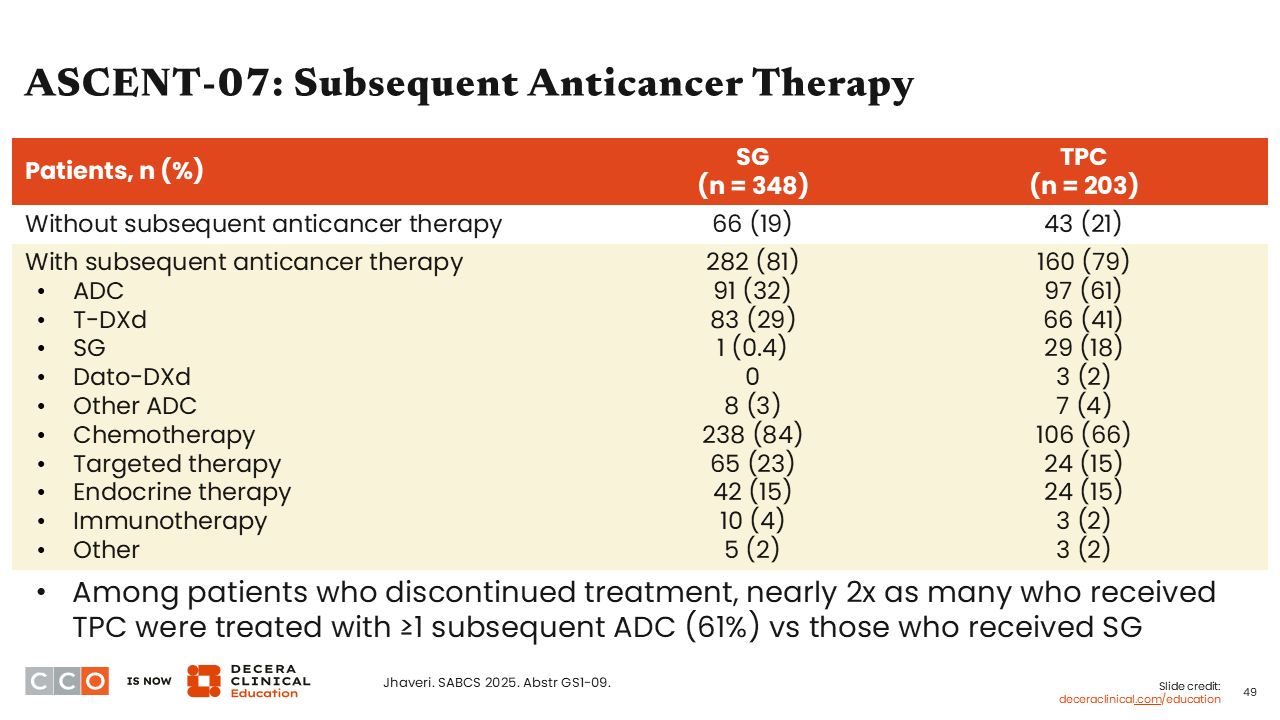
ASCENT-07: Subsequent Anticancer Therapy
Sara M. Tolaney, MD, MPH:
To better understand some of the nuances in this trial we must also note that some patients that were randomized to the TPC arm were later “pulled out” of the trial prior to confirming PFS; some of them later received subsequent treatment with an ADC. So, we can imagine how this might occur in your practice. I think that this creates bias in the study, where patients may not have centrally confirmed RECIST progression of disease but want to move on to receive ≥1 subsequent ADC (61%) that might have been available to them. I believe we saw this occurring in ASCENT-07. The investigators felt the investigator-assessed PFS that was occurring at these time points was due to new lesions that were seen, yet patients just did not meet BICR-confirmed events criterion.
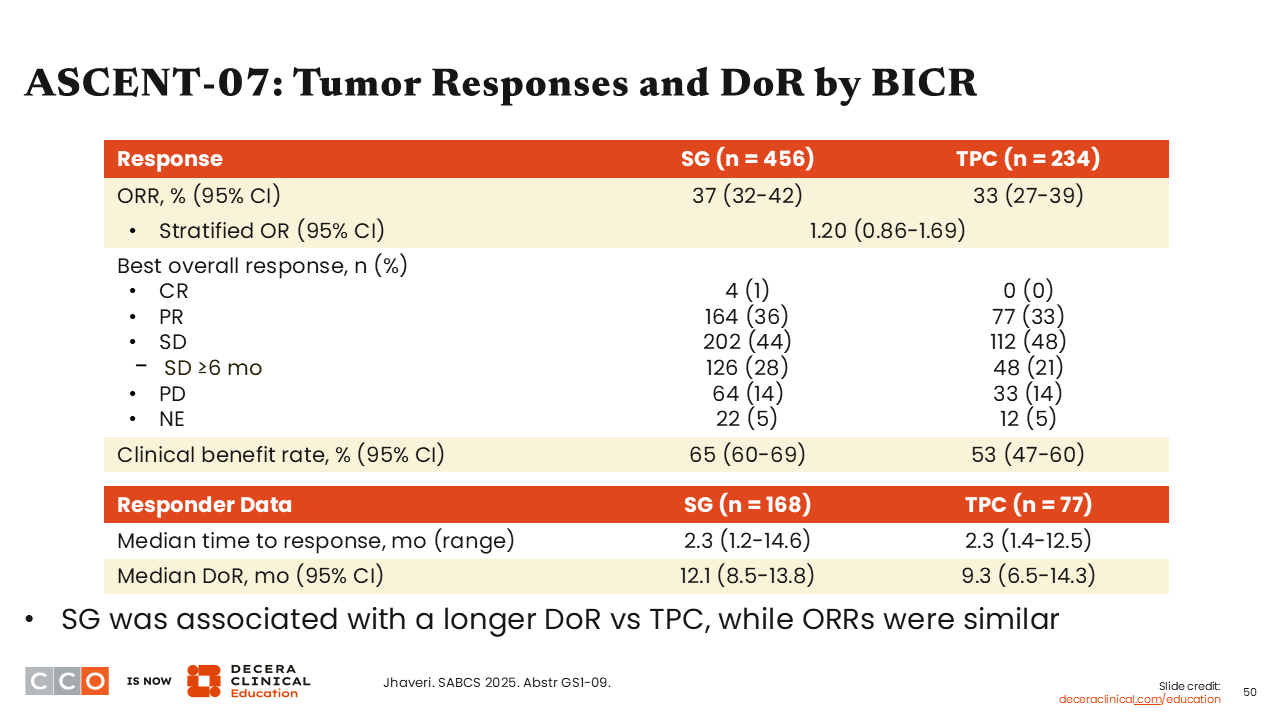
ASCENT-07: Tumor Responses and DoR by BICR
Sara M. Tolaney, MD, MPH:
We also saw that response rates were similar between the sacituzumab govitecan and TPC arms (37% vs 33%; stratified odds ratio: 1.20; 95% CI: 0.86-1.69). The median DoR was slightly longer with sacituzumab govitecan compared with TPC (12.1 vs 9.3 months).33 These results are intriguing because we continue to see a signal with sacituzumab govitecan with DoR being prolonged with this agent.
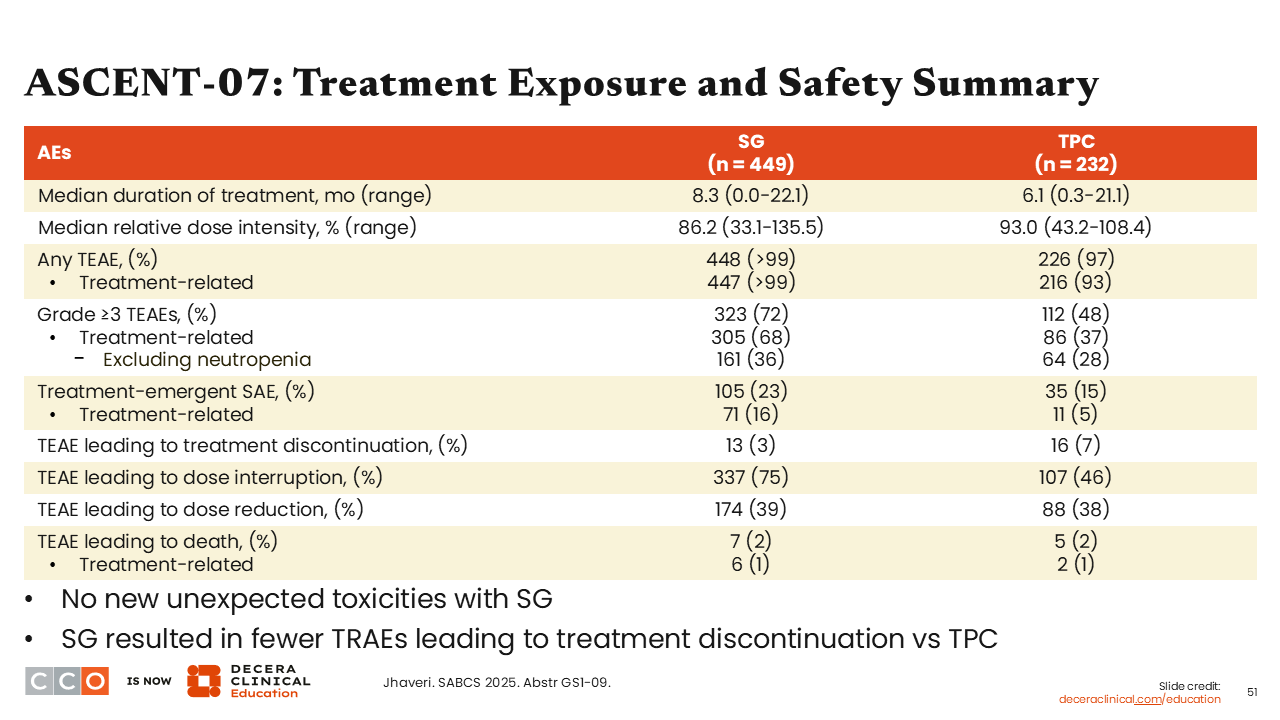
ASCENT-07: Treatment Exposure and Safety Summary
Sara M. Tolaney, MD, MPH:
Regarding treatment exposure and safety data, the treatment discontinuation rate was similar between the 2 study arms. However, there were more grade ≥3 AEs with sacituzumab govitecan vs TPC (72% vs 48%), including treatment-related but excluding neutropenia (36% vs 28%). Although dose interruptions were more common with sacituzumab govitecan vs TPC (75% vs 46%), rates of TEAEs leading to dose reductions were similar (39% vs 38%). There were 6 treatment-related deaths in the sacituzumab govitecan arm (1%) and 2 in the TPC arm (1%).
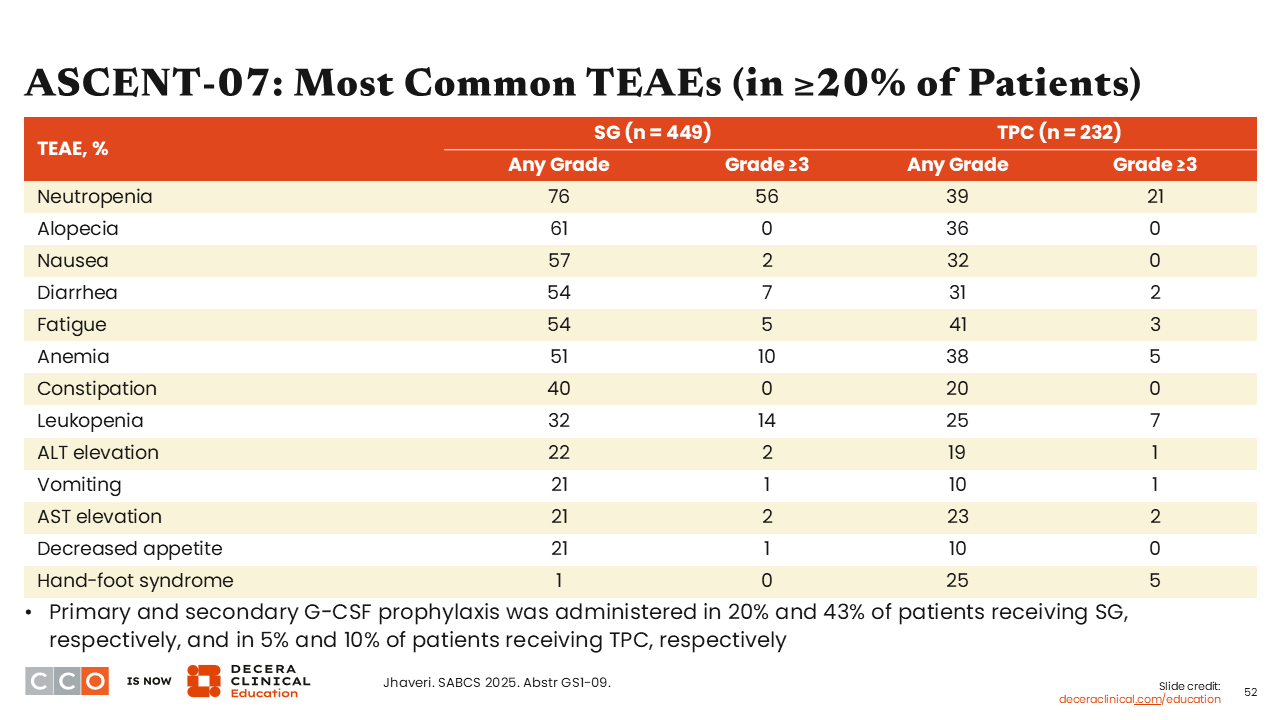
ASCENT-07: Most Common TEAEs (in ≥20% of Patients)
Sara M. Tolaney, MD, MPH:
The safety profile for sacituzumab govitecan was consistent with what we had previously observed with this agent. Because of high rates of neutropenia (grade ≥3) primary and secondary granulocyte-colony stimulating factor (G-CSF) prophylaxis was administered in 20% and 43% of patients receiving sacituzumab govitecan, respectively, and in 5% and 10% of patients receiving TPC, respectively.
My takeaway from the ASCENT study is that it does not conclude that sacituzumab govitecan is better than TPC and that it will not change current clinical practice.
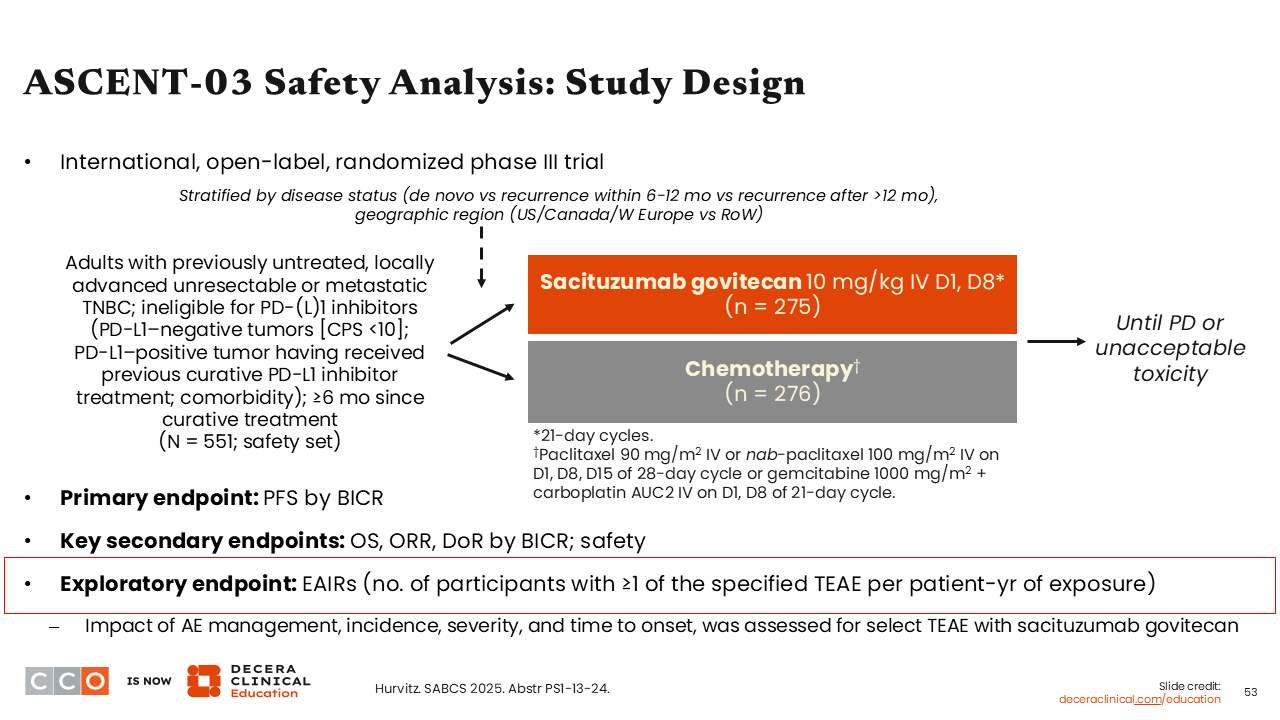
ASCENT-03 Safety Analysis: Study Design
Sara M. Tolaney, MD, MPH:
We also saw presented at SABCS 2025 the safety analyses from the phase III ASCENT-03 trial of sacituzumab govitecan vs chemotherapy in patients with previously untreated, locally advanced unresectable or metastatic TNBC who are ineligible for treatment with PD-(L)1 inhibitors.35 Efficacy data from ASCENT-03 were previously presented at the 2025 ESMO Congress which showed the study met its primary objective by improving median PFS with sacituzumab govitecan vs chemotherapy (HR: 0.62; 95% CI: 0.50-0.77; P <.001).35-37
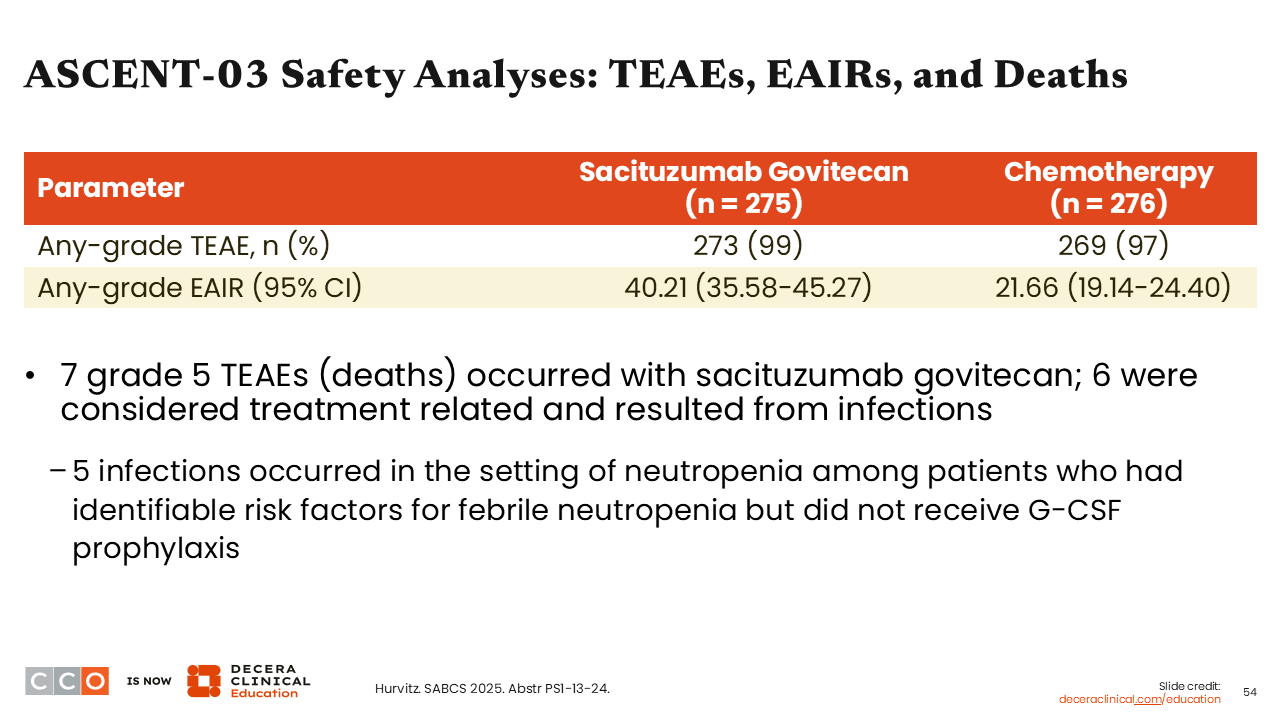
ASCENT-03 Safety Analyses: TEAEs, EAIRs, and Deaths
Sara M. Tolaney, MD, MPH:
The earlier report from ESMO36,37 showed that there were more serious AEs reported with sacituzumab govitecan compared with the chemotherapy-receiving arm, but we wondered if the rate of AEs would be similar based on adjustments for treatment exposure. Of note, on this trial, patients in the sacituzumab govitecan arm received treatment for longer because it yielded a longer duration of disease control compared with the chemotherapy arm.
One thing to keep in mind is that there were more deaths reported with sacituzumab govitecan vs chemotherapy. Of the 7 total deaths that occurred in the sacituzumab govitecan arm, 6 were considered treatment related and resulted from infection. Moreover, 5 of the 7 patients who died of infection-related complications did so without receiving prophylaxis with G-CSF, and all 5 patients met current guideline criteria for the administration of G-CSF prophylaxis.35 This is a very important reminder for us that when treating patients with sacituzumab govitecan we must consider growth factor utilization in those with prior issues related to neutropenia, with comorbidities, or of older age.
Next, we will look at the safety analysis reported at SABCS 2025 that presented the results of exposure-adjusted incidence rate of AEs.
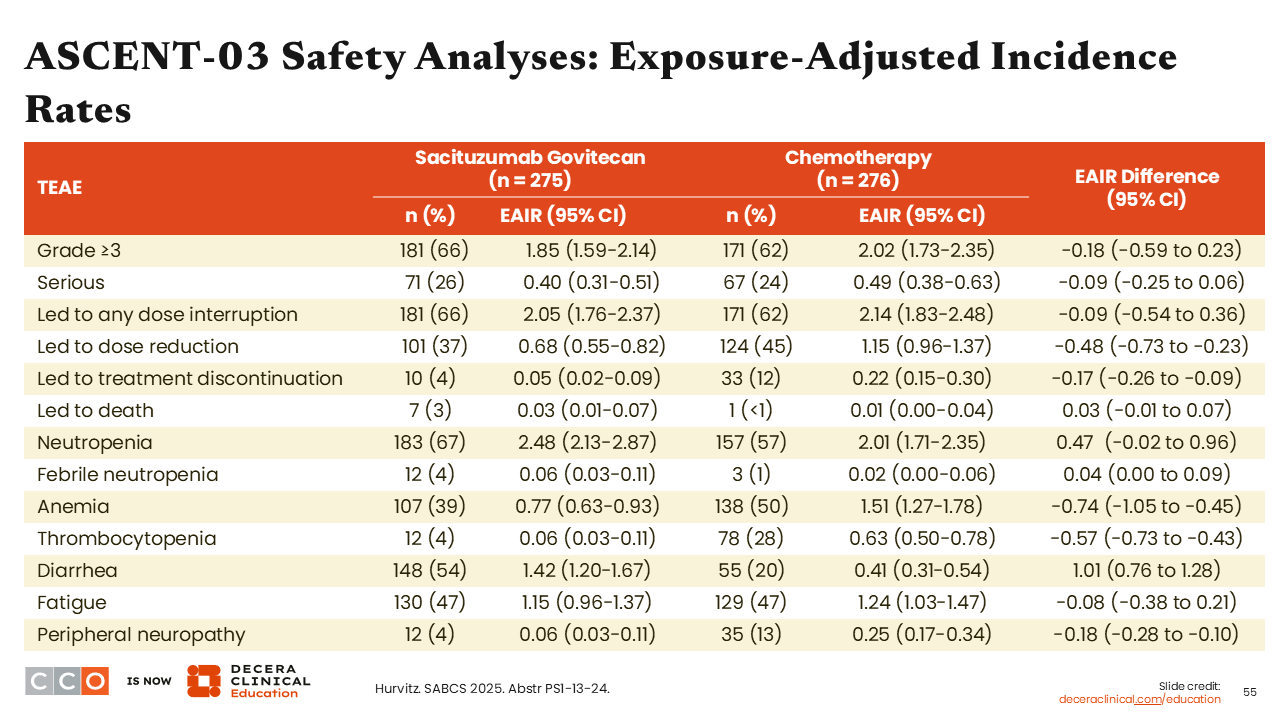
ASCENT-03 Safety Analyses: Exposure-Adjusted Incidence Rates
Sara M. Tolaney, MD, MPH:
Of interest, we saw that there were fewer events of exposure-adjusted incidence rates of thrombocytopenia (EAIR: -0.57; 95% CI: -0.73 to -0.43), anemia (EAIR: -0.74; 95%CI: -1.05 to -0.45), and peripheral neuropathy (EAIR: -0.18; 95%CI: -0.28 to -0.10) reported in the sacituzumab govitecan arm vs the chemotherapy arm. By contrast, we saw higher diarrhea with sacituzumab govitecan compared with chemotherapy (EAIR: 1.01; 95%CI: 0.76-1.28).35
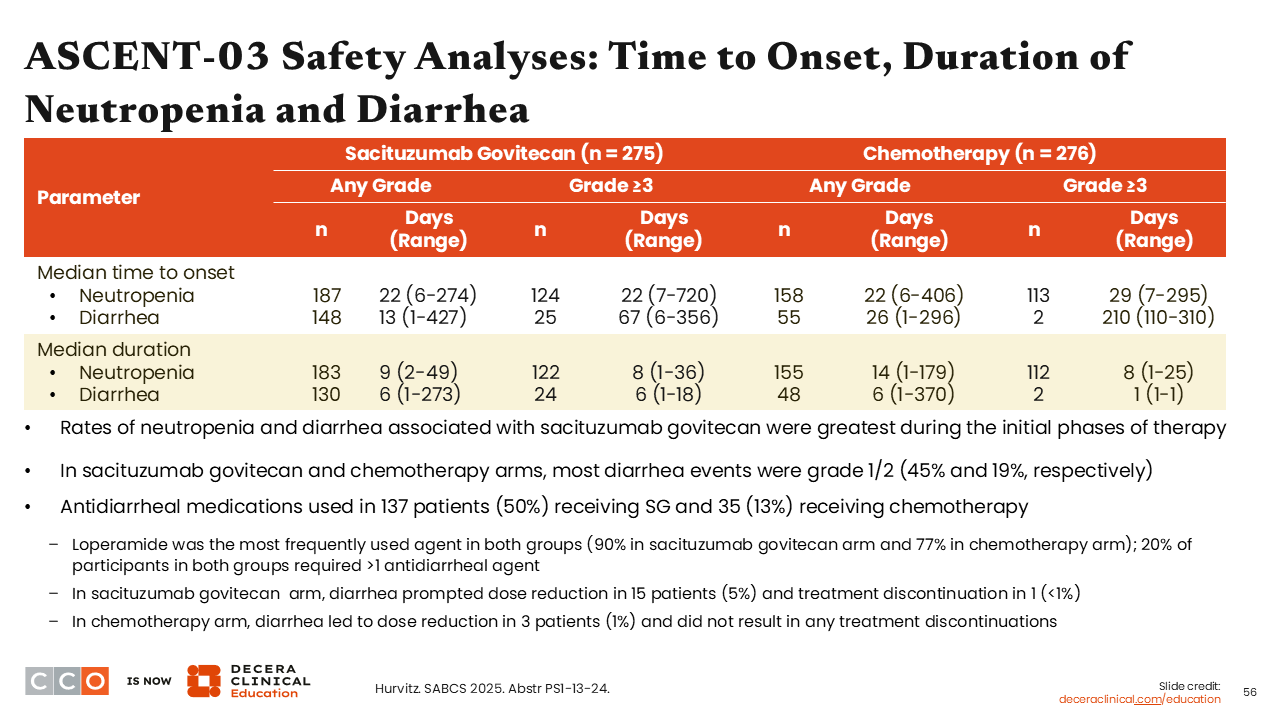
ASCENT-03 Safety Analyses: Time to Onset, Duration of Neutropenia and Diarrhea
Sara M. Tolaney, MD, MPH:
When looking at the median time (days) to any-grade neutropenia onset, we see that it was not much different between the 2 study arms (22 days [range: 6-274 days] with sacituzumab govitecan vs 22 days [range: 6-406 days] with chemotherapy). However, there was an earlier onset to any-grade diarrhea with sacituzumab govitecan compared with chemotherapy (13 days [1-427 days] vs 26 days [1-296 days]).35 Of consequence, antidiarrheal medications were used in 137 patients (50%) receiving sacituzumab govitecan and 35 patients (13%) receiving chemotherapy, with loperamide being the most frequently used agent in both groups.
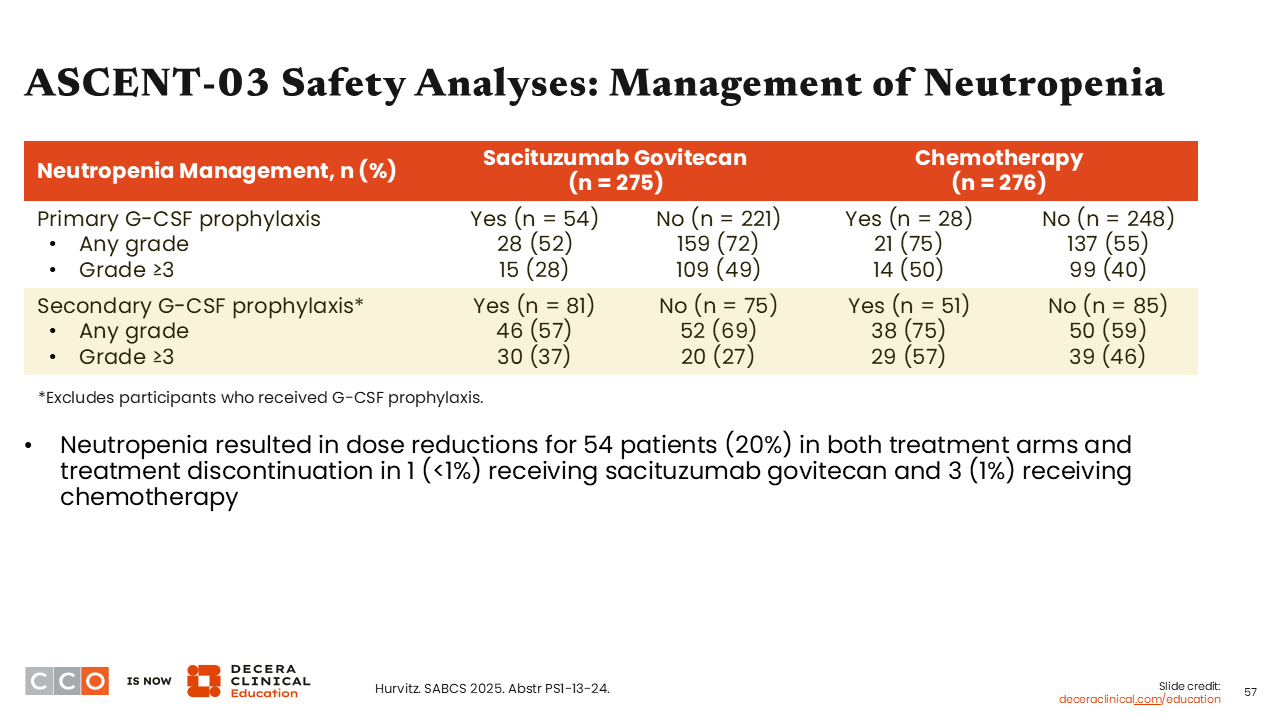
ASCENT-03 Safety Analyses: Management of Neutropenia
Sara M. Tolaney, MD, MPH:
Using an analysis of neutropenia management in the ASCENT-03 trial, it is important to note that the use of primary prophylaxis with G-CSF resulted in a decrease in neutropenia frequency and severity. Neutropenia was a common reason for dose reduction within this trial with 54 patients (20%) in both treatment arms experiencing dose reduction, and discontinuation in 1 patient (<1%) receiving sacituzumab govitecan and 3 patients (1%) receiving chemotherapy.35
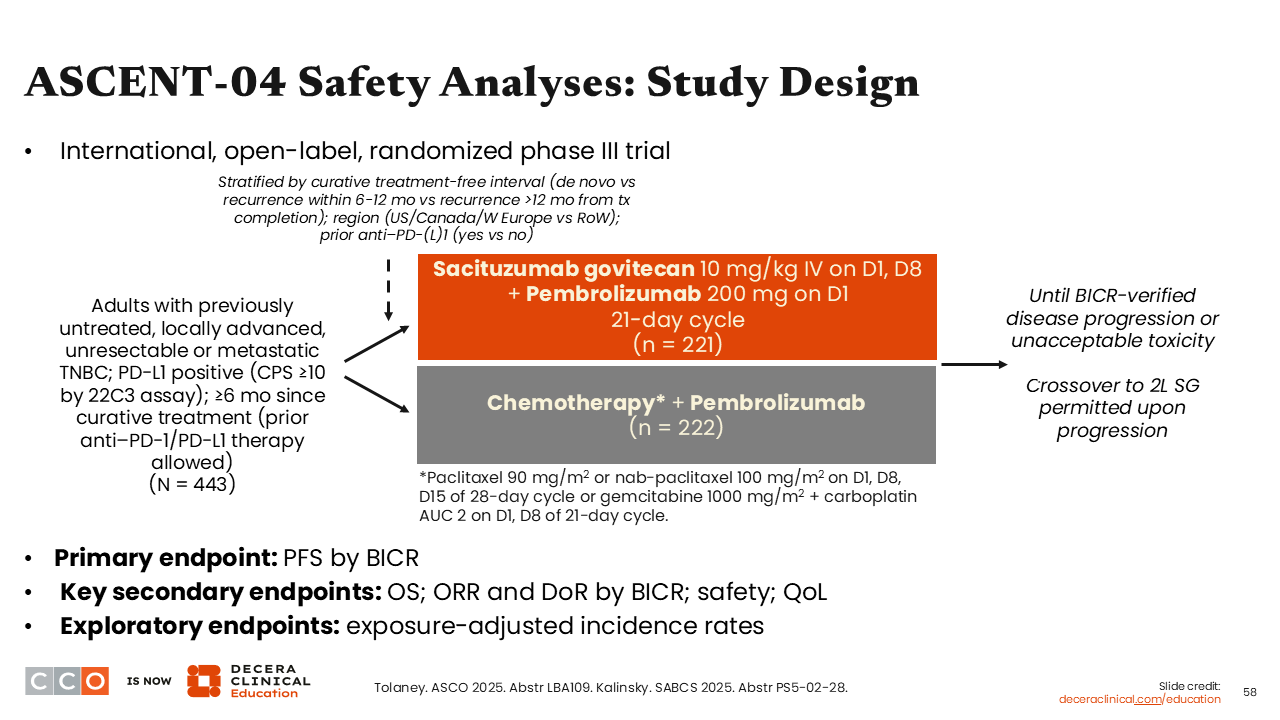
ASCENT-04 Safety Analyses: Study Design
Sara M. Tolaney, MD, MPH:
Similar to ASCENT-03, results from the phase III ASCENT-04 trial evaluating the combination of sacituzumab govitecan plus pembrolizumab vs chemotherapy plus pembrolizumab showed a significant improvement in the primary endpoint of PFS by BICR with a median PFS of 11.2 vs 7.8 months (HR: 0.65; 95% CI: 0.51-0.84) in patients with previously untreated, PD-L1–positive advanced or metastatic TNBC.38
Like the previous study, investigators from ASCENT-04 reported data from its EAIR safety analysis at SABCS 2025.39
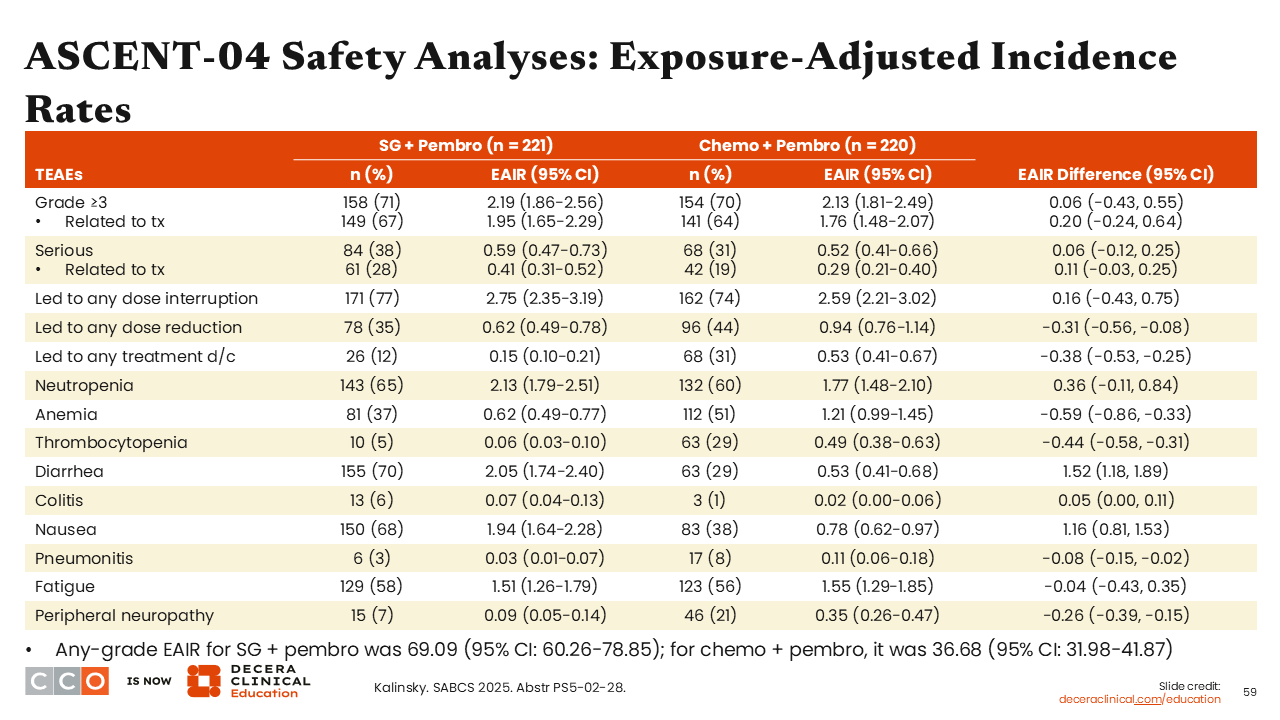
ASCENT-04 Safety Analyses: Exposure-Adjusted Incidence Rates
Sara M. Tolaney, MD, MPH:
Using EAIRs, we saw there were fewer dose reductions (EAIR: -0.31; 95% CI: -0.56 to -0.08) and discontinuations (EAIR: -0.38; 95% CI: -0.53 to -0.25) in the sacituzumab govitecan arm vs the chemotherapy arm. In addition, patients treated with sacituzumab govitecan exhibited reduced rates of anemia (EAIR: -0.59; 95% CI: -0.86 to -0.33), thrombocytopenia (EAIR: -0.44; 95% CI: -0.58 to -0.31), and peripheral neuropathy (EAIR: -0.26; 95% CI: -0.39 to -0.15) but more diarrhea (EAIR: 1.52; 95% CI: 1.18 to -1.89) and colitis (EAIR: 0.05; 95% CI: 0.00 to 0.11]) compared with those treated with chemotherapy.39
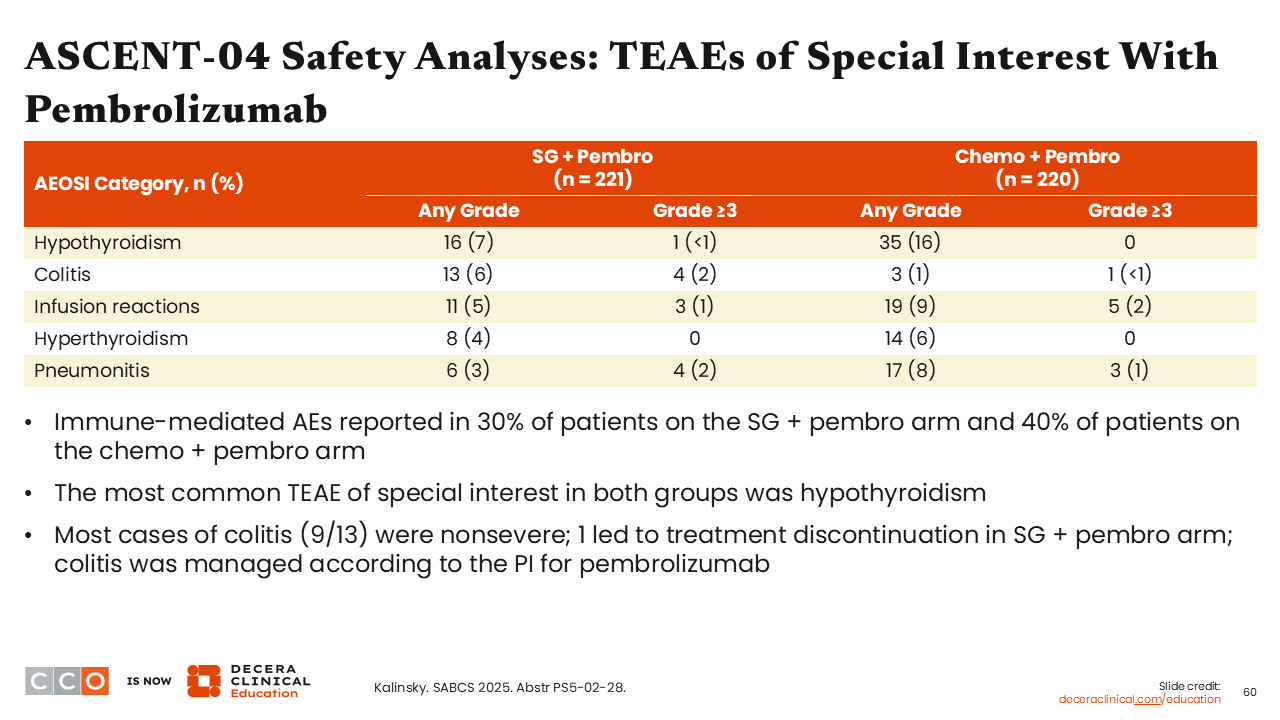
ASCENT-04 Safety Analyses: TEAEs of Special Interest With Pembrolizumab
Sara M. Tolaney, MD, MPH:
One intriguing finding was that there were fewer immune-related AEs reported in the sacituzumab govitecan plus pembrolizumab arm (30%) vs the chemotherapy plus pembrolizumab arm (40%). This is particularly reassuring because HCPs know to be vigilant about diarrhea as a common AE associated with sacituzumab govitecan. HCPs also need to be vigilant about potential worsening of colitis with the combination of sacituzumab govitecan and pembrolizumab. To this point, the incidence rate for any-grade colitis was indeed higher with the sacituzumab govitecan plus pembrolizumab combination compared with chemotherapy plus pembrolizumab (6% vs 1%).39
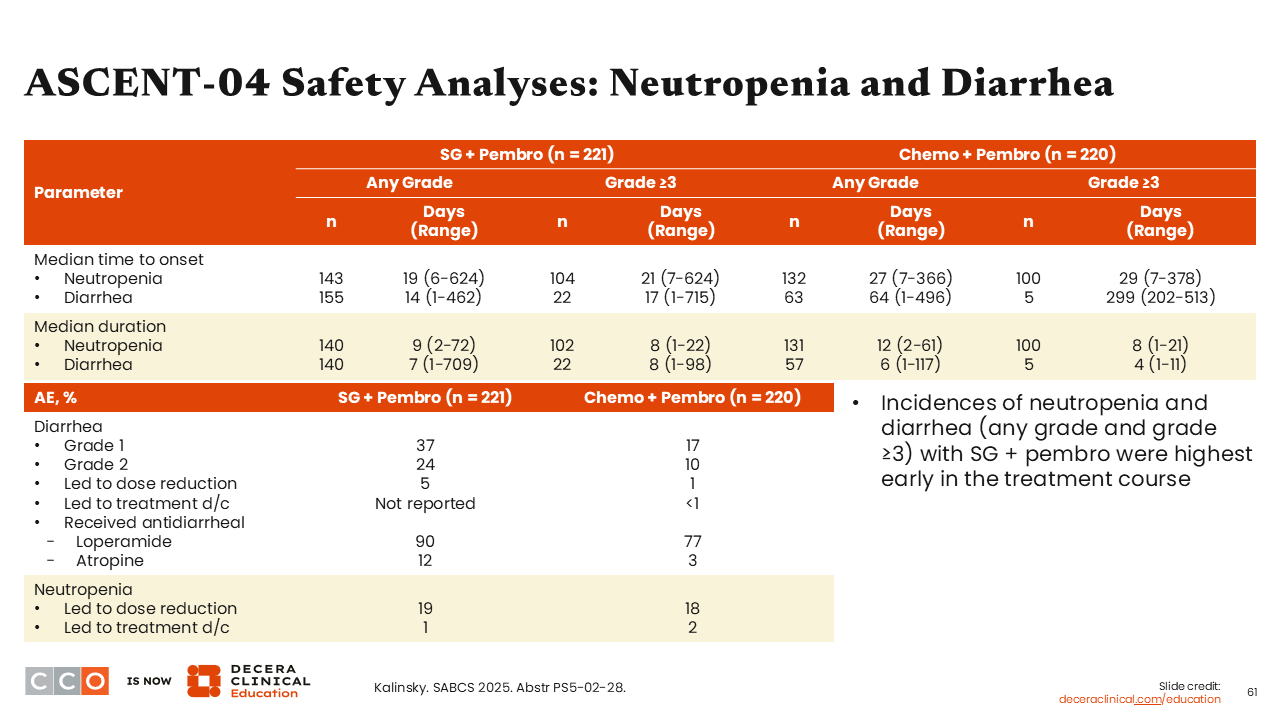
ASCENT-04 Safety Analyses: Neutropenia and Diarrhea
Sara M. Tolaney, MD, MPH:
We know that neutropenia and diarrhea are 2 common AEs associated with sacituzumab govitecan use. When looking at the median time (days) to any-grade neutropenia onset, we see that it was slightly shorter in the sacituzumab govitecan plus pembrolizumab arm at 19 days (range: 6-244 days) vs 27 days (range: 7-366 days) with chemotherapy plus pembrolizumab. However, there was a substantially earlier onset to any-grade diarrhea with sacituzumab govitecan plus pembrolizumab compared with chemotherapy plus pembrolizumab (14 days [1-462 days] vs 64 days [1-496 days]).39 Of note, no treatment discontinuations were reported in the sacituzumab govitecan plus pembrolizumab arm and fewer than 1% discontinued treatment in the chemotherapy plus pembrolizumab arm due to diarrhea. The most common agents used for the management for diarrhea in both arms were loperamide (90% and 77%, respectively) and atropine (12% and 3%, respectively).
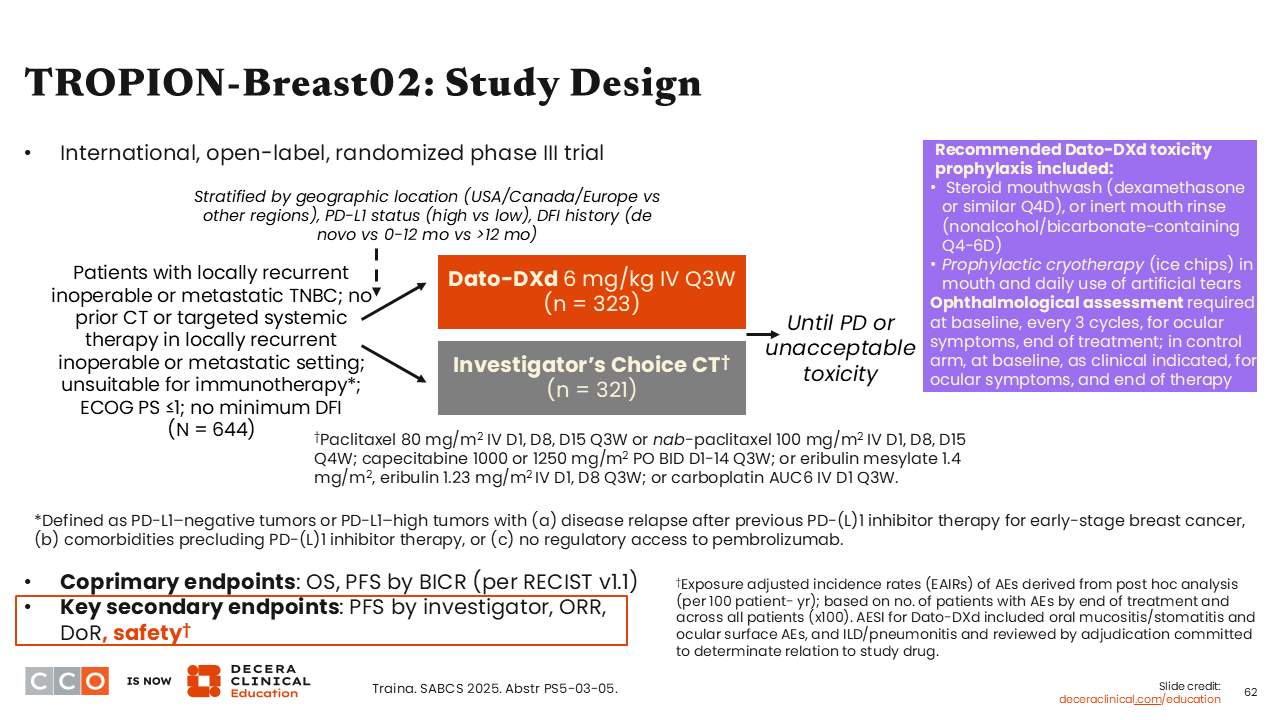
TROPION-Breast02: Study Design
Sara M. Tolaney, MD, MPH:
Next is the phase III TROPION-Breast02 trial, which compared datopotamab deruxtecan (Dato-DXd) with investigator’s choice of single-agent chemotherapy (either paclitaxel or nab-paclitaxel; capecitabine; eribulin or eribulin mesylate; or carboplatin) in previously untreated patients with locally recurrent inoperable or metastatic TNBC ineligible for immunotherapy. Primary results were first presented at the 2025 ESMO Congress showing that Dato-DXd led to improved median PFS (10.8 vs 5.6 months; HR: 0.57; 95% CI: 0.47-0.69; P <.0001) and median OS (23.7 vs 18.7 months; HR: 0.79; 95% CI: 0.64-0.98; P <.0291) compared with investigator’s choice of chemotherapy.40
At SABCS 2025, trial investigators presented data from TROPION-Breast02 that specifically focused on the safety analysis.41
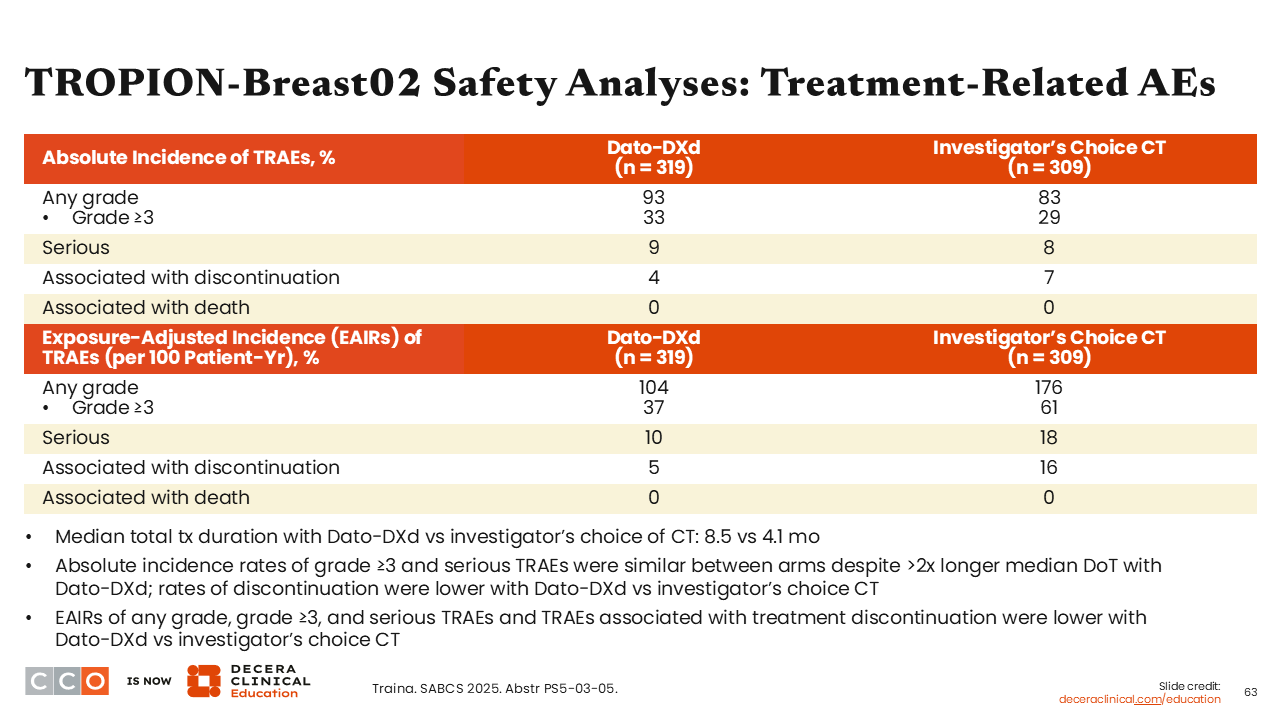
TROPION-Breast02 Safety Analyses: Treatment-Related AEs
Sara M. Tolaney, MD, MPH:
At SABCS, investigators reported that rates for any-grade (93% vs 83%), grade ≥3 (33% vs 29%), and serious (9% vs 8%) AEs were similar between the Dato-DXd and the investigator’s choice of chemotherapy arm. There were fewer treatment-related discontinuations in the Dato-DXd arm vs the chemotherapy arm (5% vs 16%), and median total treatment duration with Dato-DXd vs investigator’s choice of chemotherapy was 8.5 months compared with 4.1 months.41
Regarding EAIRs of grade ≥3 (37% vs 61%), serious treatment-related AEs (10% vs 18%), and treatment-related AEs associated with treatment discontinuation (5% vs 16%) were all lower with Dato-DXd vs investigator’s choice of chemotherapy.
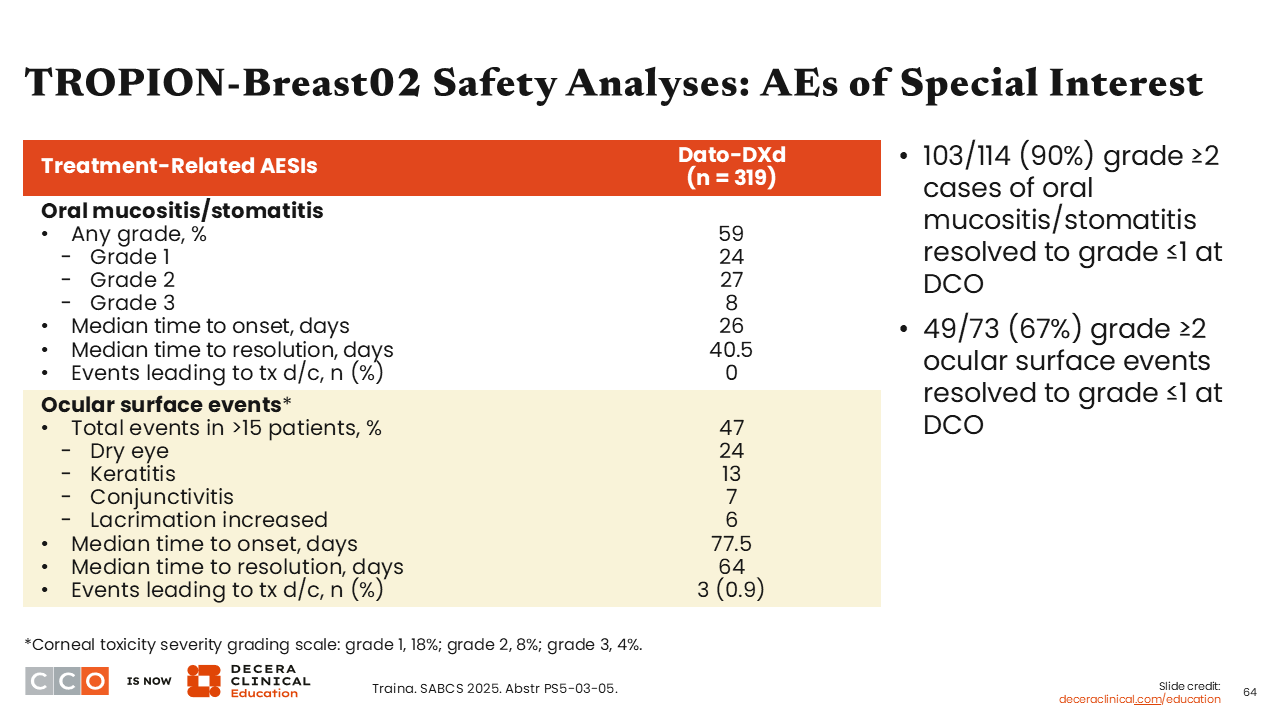
TROPION-Breast02 Safety Analyses: AEs of Special Interest
Sara M. Tolaney, MD, MPH:
Two important AEs to keep in mind with Dato-DXd are mucositis/stomatitis and ocular surface events. Any- grade mucositis/stomatitis occurred in approximately 60% of patients in the Dato-DXd arm (grade 3: 8%). These AEs occurred despite the recommended use of prophylaxis with steroid mouthwash or inert mouth rinse and ice chips. Overall, among patients who experienced grade 2 or greater events of mucositis/stomatitis, 90% were resolved to grade ≤1 by the time of data cutoff.
Regarding ocular events, approximately 50% of patients receiving Dato-DXd experienced ocular surface events including dry eyes (24%), keratitis (13%), conjunctivitis (7%), and lacrimation increase (6%). The time to onset and time to resolution for mucositis/stomatitis were 26 days and 40.5 days, respectively, and for ocular surface events were 77.5 days and 64.0 days, respectively. Of note, ocular surface events leading to treatment discontinuation occurred in 3 (<1%) patients.
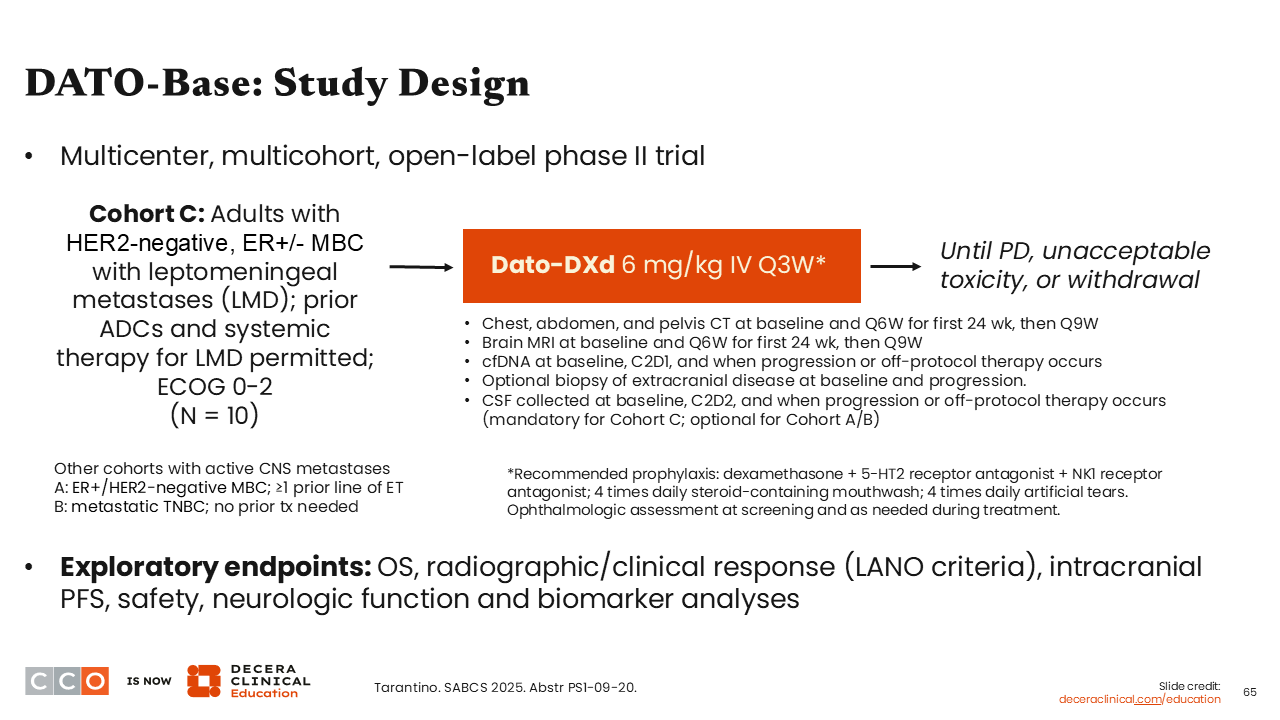
DATO-Base: Study Design
Sara M. Tolaney, MD, MPH:
Finally, an interesting single-arm trial presented at SABCS 2025 for Dato-DXd was the phase II DATO-Base (Cohort C) in patients with HER2-negative, ER-positive or ER-negative MBC with leptomeningeal disease (LMD). The trial has 2 other cohorts not presented in this analysis including patients with active brain metastases and ER-positive/HER2-negative MBC and at least 1 prior line of ET (cohort A) and patients with metastatic TNBC and no prior treatment needed for enrollment (cohort B).41
Because of the known toxicities associated with Dato-DXd, this study also recommends prophylaxis with dexamethasone plus a 5-HT2 receptor antagonist and NK1 receptor antagonist, 4 times daily steroid-containing mouthwash, and 4 times daily artificial tears. Ophthalmologic assessment was performed at screening and as needed during treatment.
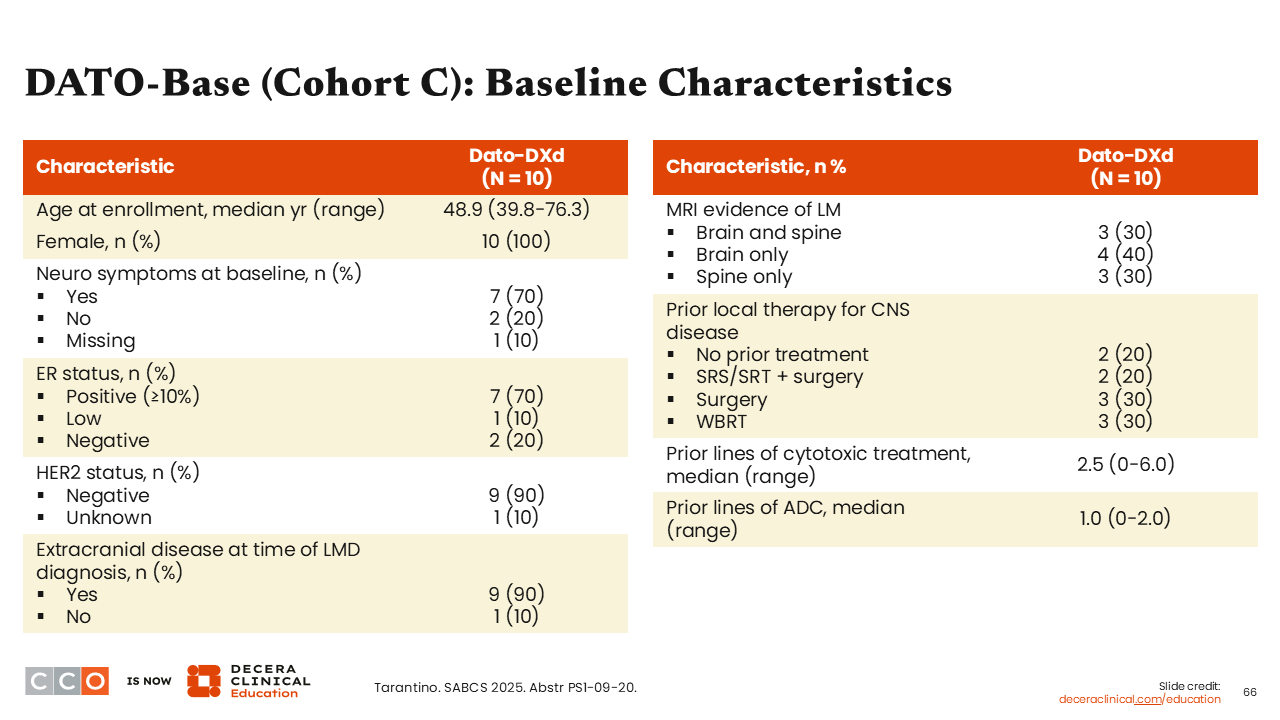
DATO-Base: Baseline Characteristics
Sara M. Tolaney, MD, MPH:
Data are reported for the 10 patients who were enrolled in cohort C and who received treatment with Dato-DXd. Positive ER status (≥10%) was reported in 70% of patients. These patients could have received prior treatment with an ADC (median prior lines of ADC was 1). Median age at enrollment was 48.9 years (range 39.8-76.3), all were female, and 70% reported neurologic symptoms. Extracranial disease at the time of diagnosis was 90% with magnetic resonance imaging evidence of LMD in brain and spine (30%), brain only (40%), and spine only (30%) documented.
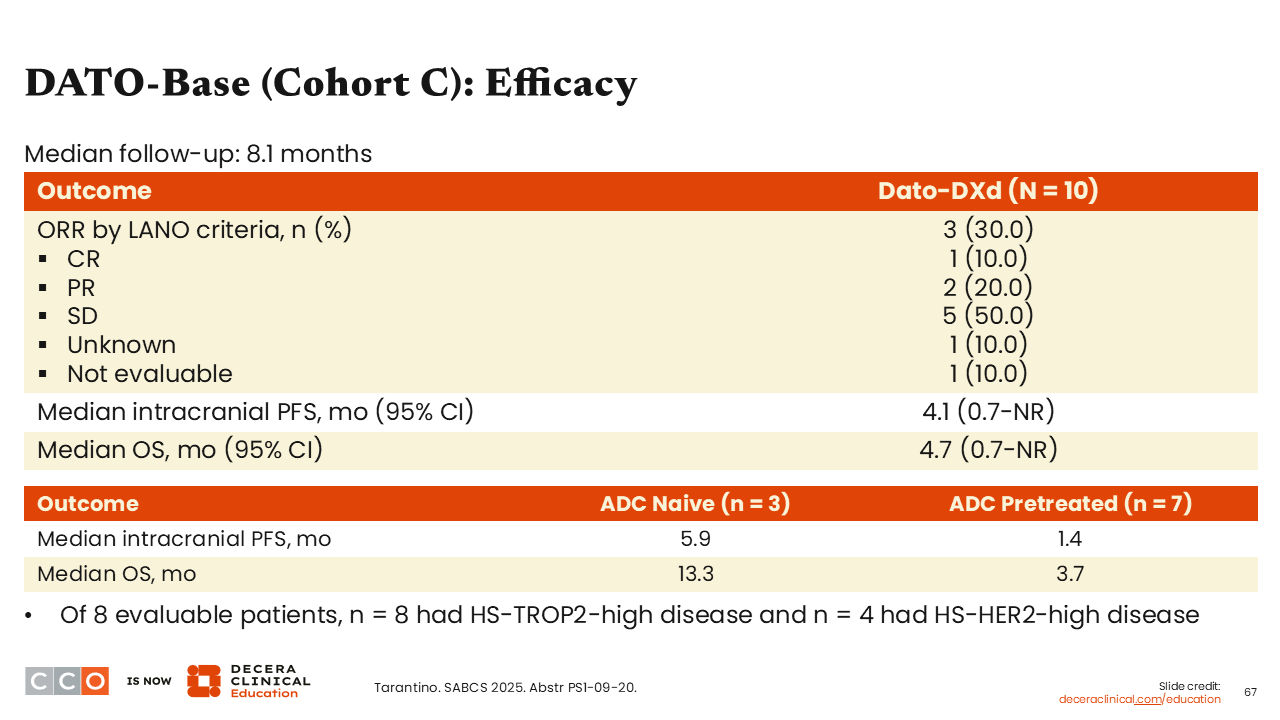
DATO-Base (Cohort C): Efficacy Summary
Sara M. Tolaney, MD, MPH:
Overall response was 30.0%, the median intracranial PFS was 4.1 months, and the median OS was 4.7 months. Although these numbers are quite small, 7 patients (70%) had prior ADC treatment and 3 were ADC naive. As one should expect, the ADC-naive patients had a much longer median intracranial PFS and OS (5.9 months and 13.3 months, respectively) vs those who were pretreated with ADC therapy (1.4 months and 3.7 months, respectively).
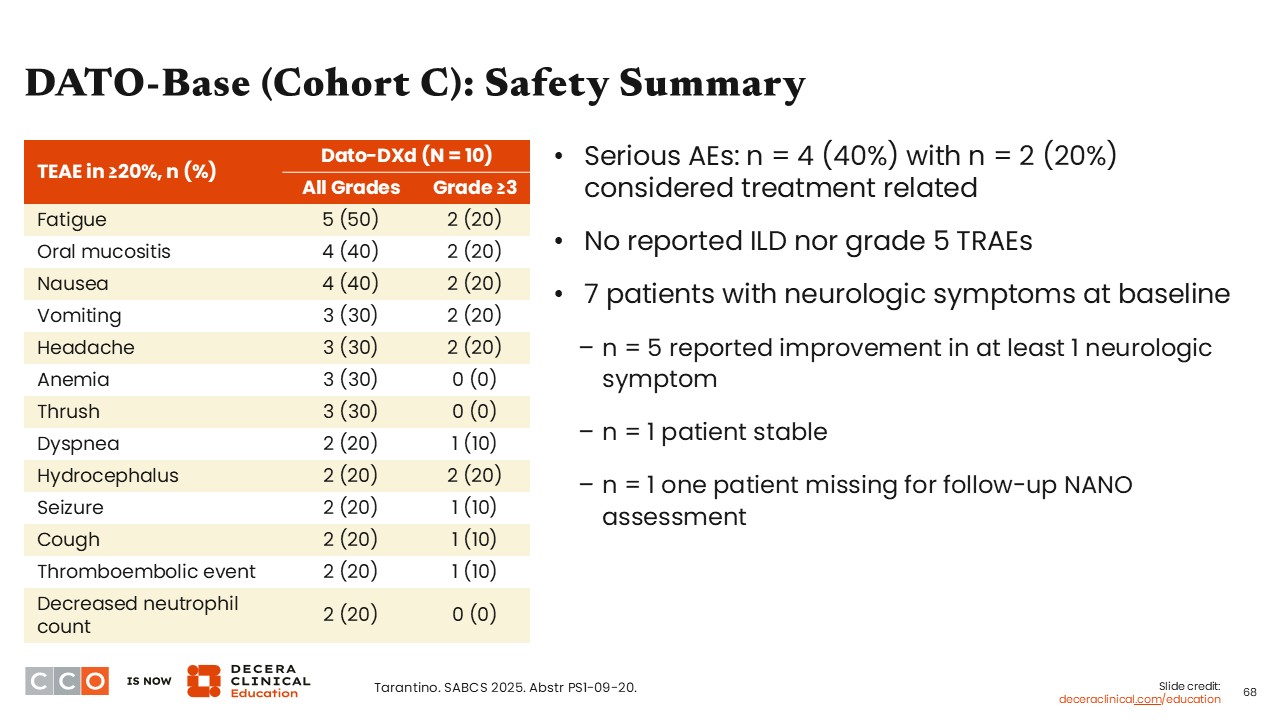
DATO-Base (Cohort C): Safety Summary
Sara M. Tolaney, MD, MPH:
Regarding the safety of Dato-DXd in this relatively small number of patients, we see data are consistent with previous Dato-DXd safety profile with the most common all-grade AEs including fatigue (50%), oral mucositis (40%), nausea (40%), vomiting, headache and anemia (both 30%). The most common grade ≥3 AEs were fatigue, oral mucositis, nausea, vomiting, headache, and hydrocephalus— all seen in at least 2 patients (20%).