CME
Decera Clinical Education Independent Conference Highlights of the San Antonio Breast Cancer Symposium 2025: Advanced and Metastatic Breast Cancer
Physicians: Maximum of 0.50 AMA PRA Category 1 Credit™
Released: February 20, 2026
Expiration: August 19, 2026
Activity
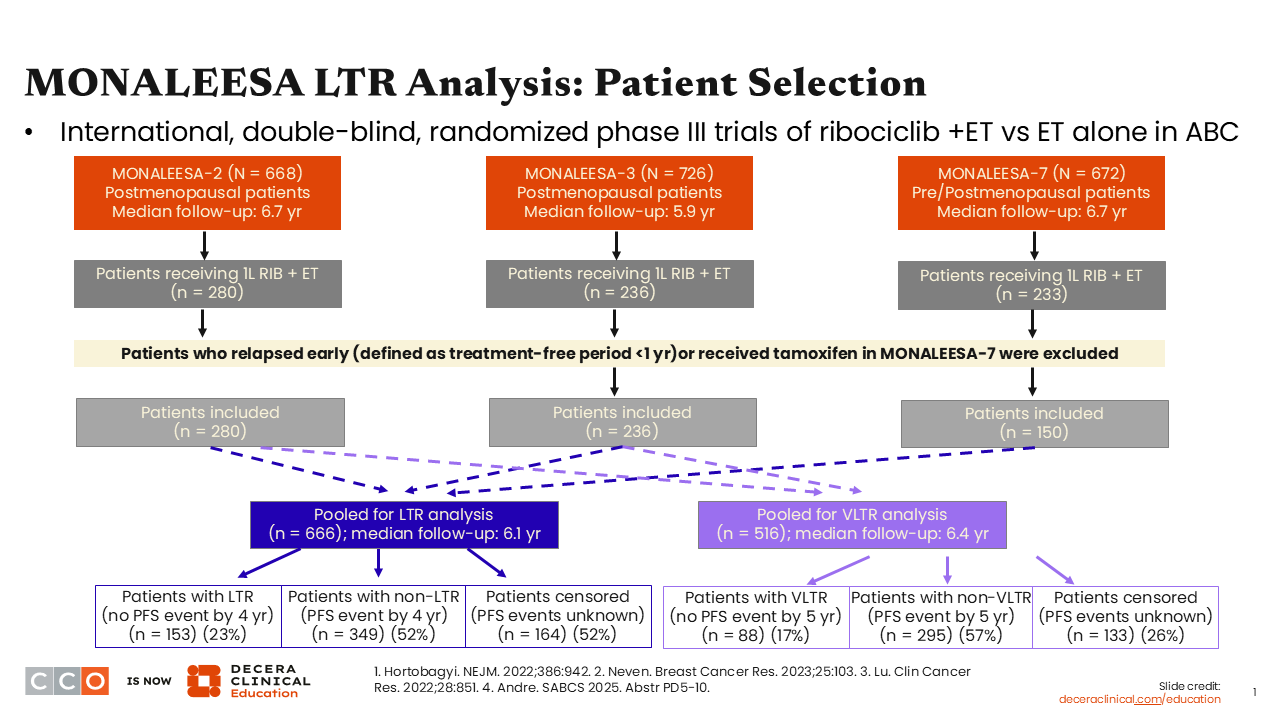
MONALEESA LTR Analysis: Patient Selection
Erica L. Mayer, MD, MPH, FASCO:
I would like to start by sharing recent clinical trial updates for the first-line setting that were presented at 2025 SABCS.
For most patients globally, the SoC for hormone receptor (HR)–positive MBC in the first-line setting is ET with a CDK4/6 inhibitor.1 I have seen many patients who do extraordinarily well with this regimen and have years of disease control. Yet it can be challenging to know in advance which patients are going to experience LTR and who might have earlier disease progression.
A pooled analysis of the MONALEESA trials was designed to identify predictors of LTR (>4 years of disease control) or VLTR (>5 years). As a reminder, the trials that were included in this pooled analysis were MONALEESA-2, -3, and -7. These were large, phase III trials that evaluated ET with or without ribociclib for HR-positive, HER2-negative ABC.2-4
In looking at the initial outcomes, the investigators found that 23% of patients had LTR and 17% had VLTR. The pooled analysis focused on these patients with very good benefits.5
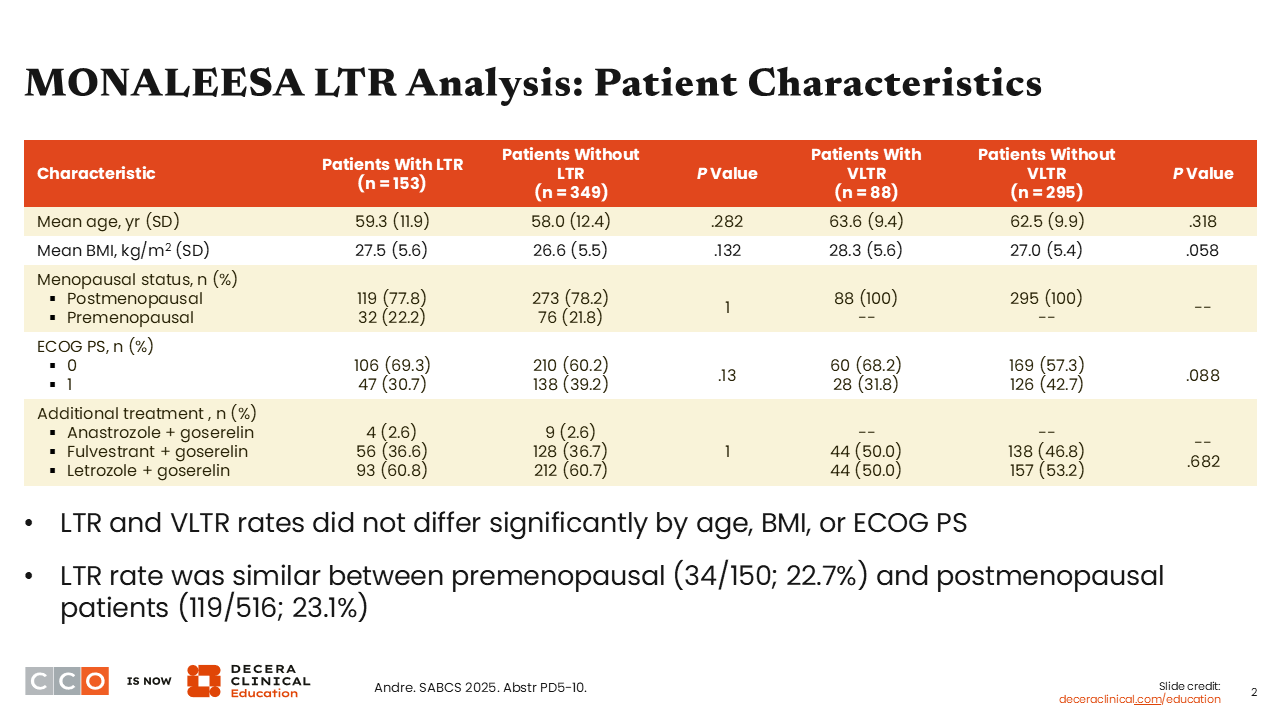
MONALEESA LTR Analysis: Patient Characteristics
Erica L. Mayer, MD, MPH, FASCO:
Baseline characteristics included in the pooled analyses show that, in general, patients with LTR or VLTR did not differ significantly from those without a LTR or VLTR by age (P = .282 and .318), body mass index (P = .132 and .058), or Eastern Cooperative Oncology Group (ECOG) performance status (P = .130 and .088). In addition, the LTR rate was similar between patients who were premenopausal vs postmenopausal (P = 1.0).5 We must also remember that premenopausal patients all receive treatment with an LHRH agonist as well.6
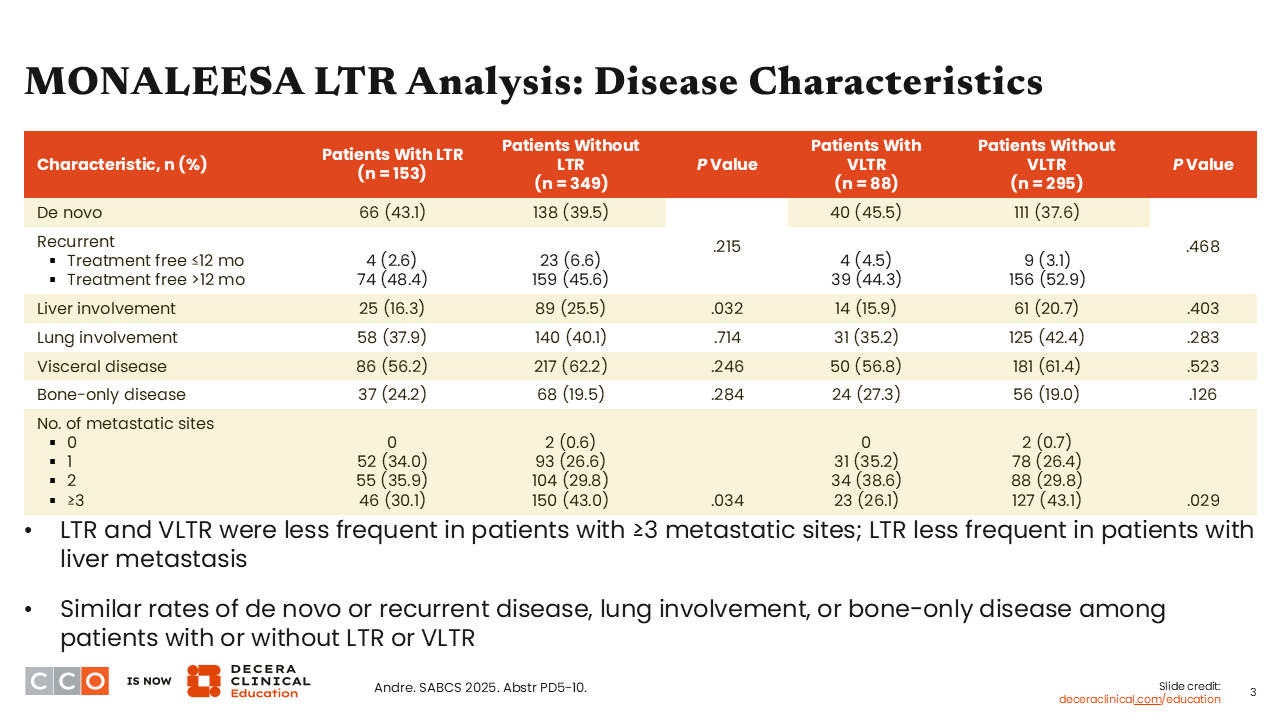
MONALEESA LTR Analysis: Disease Characteristics
Erica L. Mayer, MD, MPH, FASCO:
Now let’s look at which disease characteristics may be predictive of patients who will achieve LTR or VLTR vs those who did not achieve durable responses. Findings from the pooled analysis suggest a greater likelihood of disease control in those with fewer metastatic sites. Patients with 3 or more metastatic sites, including those with liver metastases, were less likely to achieve LTR or VLTR (P = .034 and .029, respectively). Of importance, there were similar rates of LTR and VLTR in patients with de novo or recurrent disease (P = .215 and .468), lung involvement (P = .714 and .283), or bone-only disease (P = .284 and .126). This is interesting because often healthcare professionals (HCPs) think about these categories for identifying patients who might do better but based on data from this pooled analysis, they all did similar. I would also add that responses were balanced whether patients had a treatment-free interval of >12 months (+LTR+ vs -LTR: 48.4% vs 45.6%; +VLTR vs -VLTR: 44.3% vs 52.9%) or ≤12 months (+LTR+ vs -LTR: 2.6% vs 6.6%; +VLTR vs – VLTR: 4.5% vs 3.1%).5
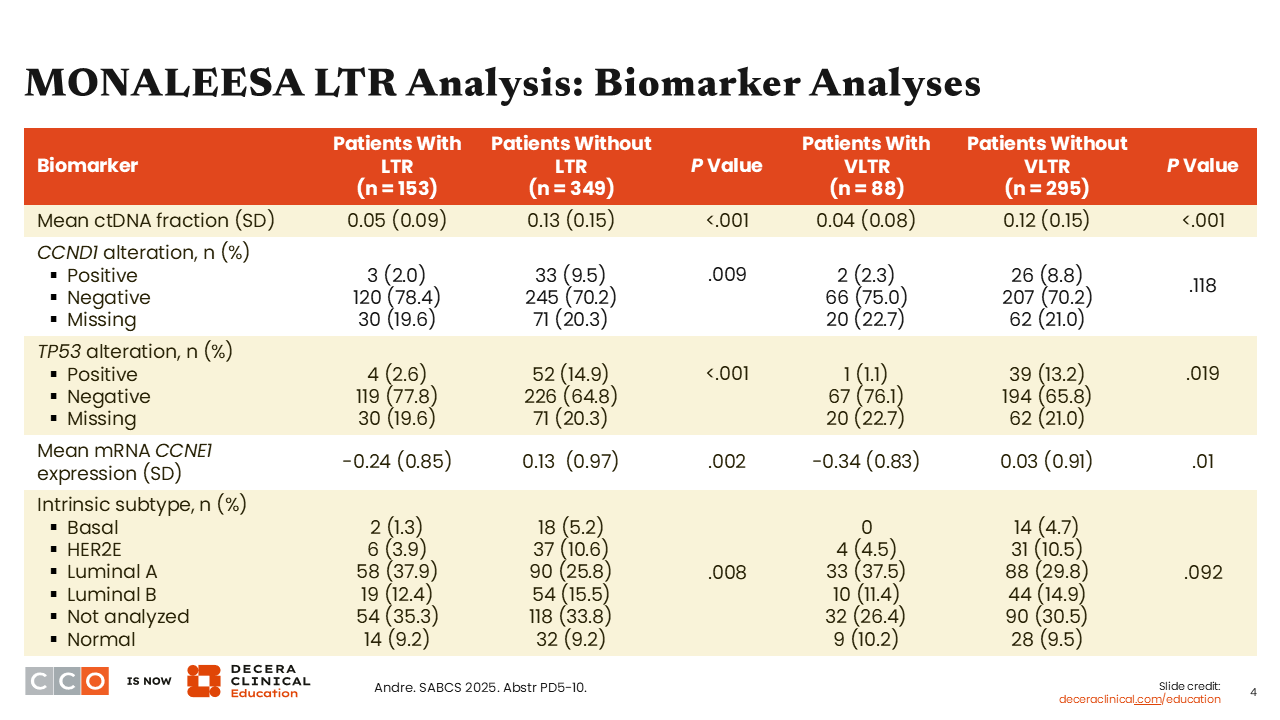
MONALEESA LTR Analysis: Biomarker Analyses
Erica L. Mayer, MD, MPH, FASCO:
Shifting our attention to biomarkers, an expansive analysis was done to look at molecular predictors of LTR or VLTR. In general, the results are in alignment with what most HCPs think of as biologic predictors of poor response. For example, it was reported that patients who carry a TP53 gene alteration (“positive”) were less likely to achieve LTR and VLTR (P = <.001 and .019), and that patients with less expression of CCNE1 (P = .002) were more likely to have LTR. Patients with luminal A subtype also saw improvements in terms of LTR (P = .008) and VLTR (P = .092).
The biologic features that generally suggest favorable tumor biology were aligned with patients who experienced prolonged disease control. Thus, what we can take away from this pooled analysis from MONALEESA-2, -3, and -7 is that there are certain clinical pathologic features—decreased burden of metastatic disease, less liver involvement, and molecular features like TP53 negative or nonbasal subtype—that are predictive of those who will do well on and have longer periods of disease control with first-line ET and a CDK4/6 inhibitor.5
ELEVATE: Study Design
Erica L. Mayer, MD, MPH, FASCO:
Now I want to move on to a hot topic in the management of ER-positive, HER2-negative locally-advanced or MBC: oral selective estrogen receptor degraders (SERDs). This is a very active area of research and clinical interest, with 4 lead oral SERD therapies currently in clinical development. These oral therapies are designed to target the ER, decreasing expression of estrogen responsive elements and leading to the degradation of the receptor itself. This is a profound way to target the ER, in contrast to an AI, for example, which only aims to deplete the body of available estrogen.
Furthermore, oral SERDs may have preferential activity in the setting of ESR1 mutation, which can lead to constitutive activation of the ER and is a mechanism of resistance to ET.7 I am going to provide a brief summary of the latest data on oral SERDs.
Elacestrant is an FDA-approved oral SERD indicated as a monotherapy for postmenopausal patients with ER-positive, HER2-negative, ESR1 mutated ABC or MBC and disease progression after 1 or more prior line of ET.8
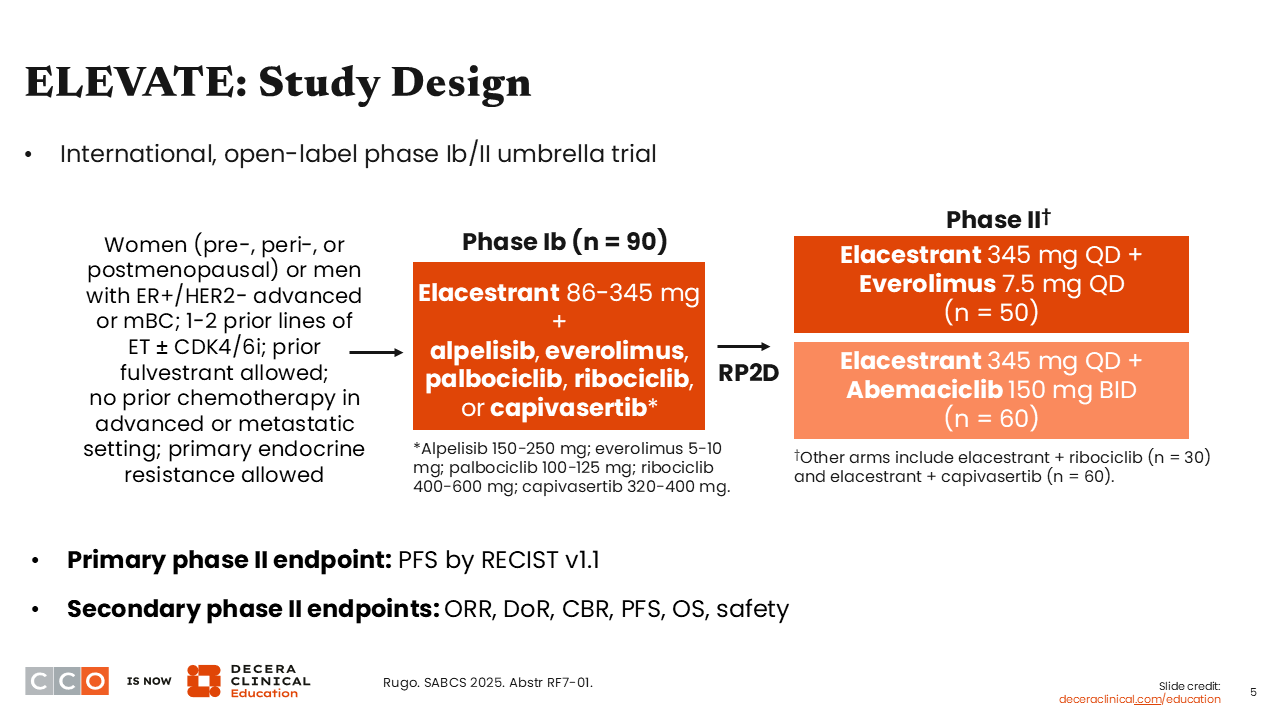
At SABCS 2025, we saw a report for the international, open-label, phase II portion of the ELEVATE umbrella trial evaluating elacestrant plus everolimus (n = 50) or elacestrant with abemaciclib (n = 60) in patients with ER-positive/HER2-negative locally advanced or MBC (NCT05563220). Patients enrolled in the study had pretreated disease. They could have 1-2 prior lines of ET with or without a CDK4/6 inhibitor and previous treatment with fulvestrant, but prior chemotherapy in the advanced or metastatic setting was not allowed. The primary endpoint was PFS by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.9
ELEVATE: Baseline Characteristics
Erica L. Mayer, MD, MPH, FASCO:
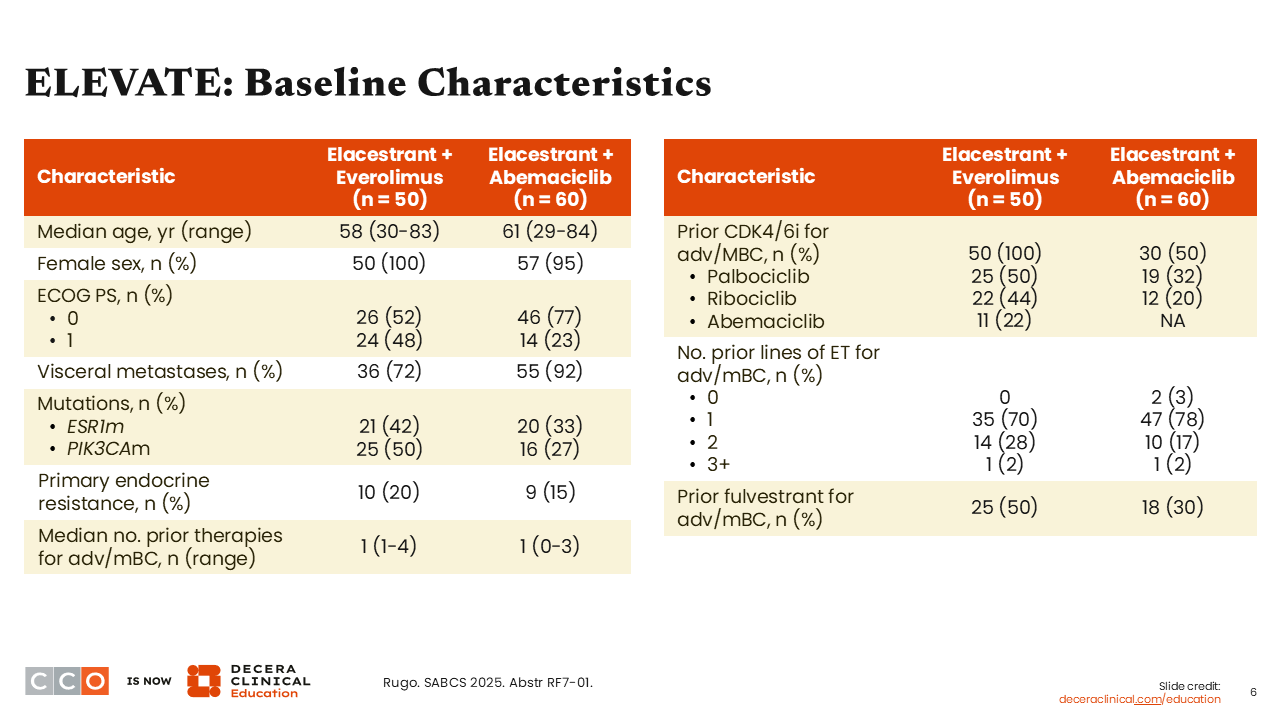
Baseline characteristics of these 2 cohorts (elacestrant with everolimus and elacestrant with abemaciclib) show most patients (72% and 92%) had visceral metastases. The majority also had a median of 1 prior line of ET for advanced or metastatic disease (70% and 78%, respectively). There was some imbalance between cohorts with patients in the everolimus arm compared with the abemaciclib arm having received previous exposure to CDK4/6 inhibitors (previous palbociclib 50% vs 32%; previous ribociclib: 44% vs 20%; previous abemaciclib: 22% vs NA). In other words, all the patients in the everolimus arm had prior treatment with a CDK4/6 inhibitor vs only half of patients in the abemaciclib arm. Moreover, 50% of patients in the everolimus arm vs 30% in the abemaciclib arm had prior treatment with fulvestrant.9
ELEVATE: PFS in All Participants and Key Subgroups
Erica L. Mayer, MD, MPH, FASCO:
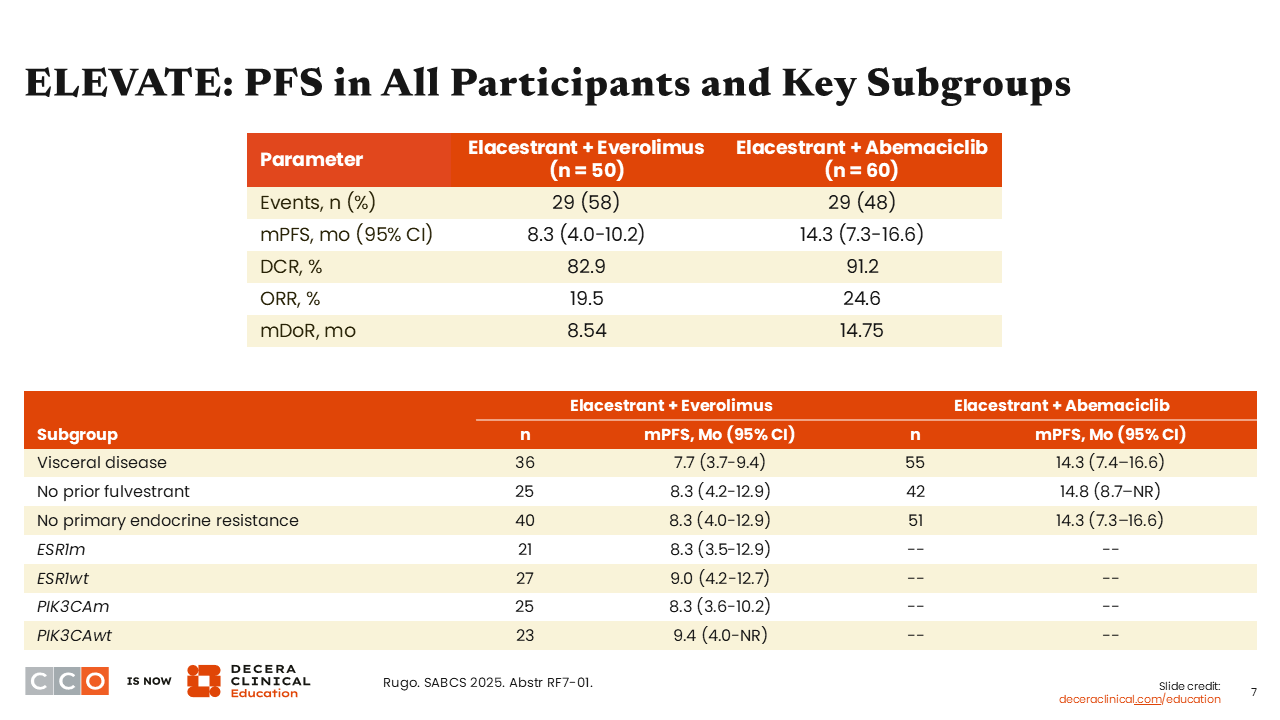
In the elacestrant plus everolimus arm, the median PFS was 8.3 months (95% CI: 4.0-10.2), disease control rate (DCR) was 82.9%, overall response rate (ORR) was 19.5%, and median duration of response (DoR) was 8.54 months. In the elacestrant plus abemaciclib arm, median PFS was 14.3 months (95% CI: 7.3-16.6), DCR was 91.2%, ORR was 24.6%, and mDoR was 14.75 months.
The benefit of combining elacestrant with everolimus was seen consistently across subgroups, including in patients with an ESR1 or PIK3CA mutation.
The benefit with elacestrant plus abemaciclib was preserved in patients with visceral disease, no prior fulvestrant therapy, and no prior endocrine resistance. Outcomes in patients by ESR1 or PIK3CA mutation status were not presented for this cohort.
To date, in the various trials combining oral SERDs with a targeted partner we see encouraging PFS with data almost always reaching more than 6 months of disease control. In these analyses from ELEVATE, the combination of elacestrant plus abemaciclib leads to a remarkable 14-month median PFS. It is important to note that approximately half of these patients had not previously received a CDK4/6 inhibitor, therefore we should expect a robust response if the disease had not been treated with this very active class of antineoplastic agents.9
ELEVATE: Safety of Elacestrant + Everolimus
Erica L. Mayer, MD, MPH, FASCO:
An important consideration with the use of an oral SERD in combination with a targeted agent is that oral SERDs are an extraordinarily well-tolerated drug class and as such contribute little toxicity to the patient experience.
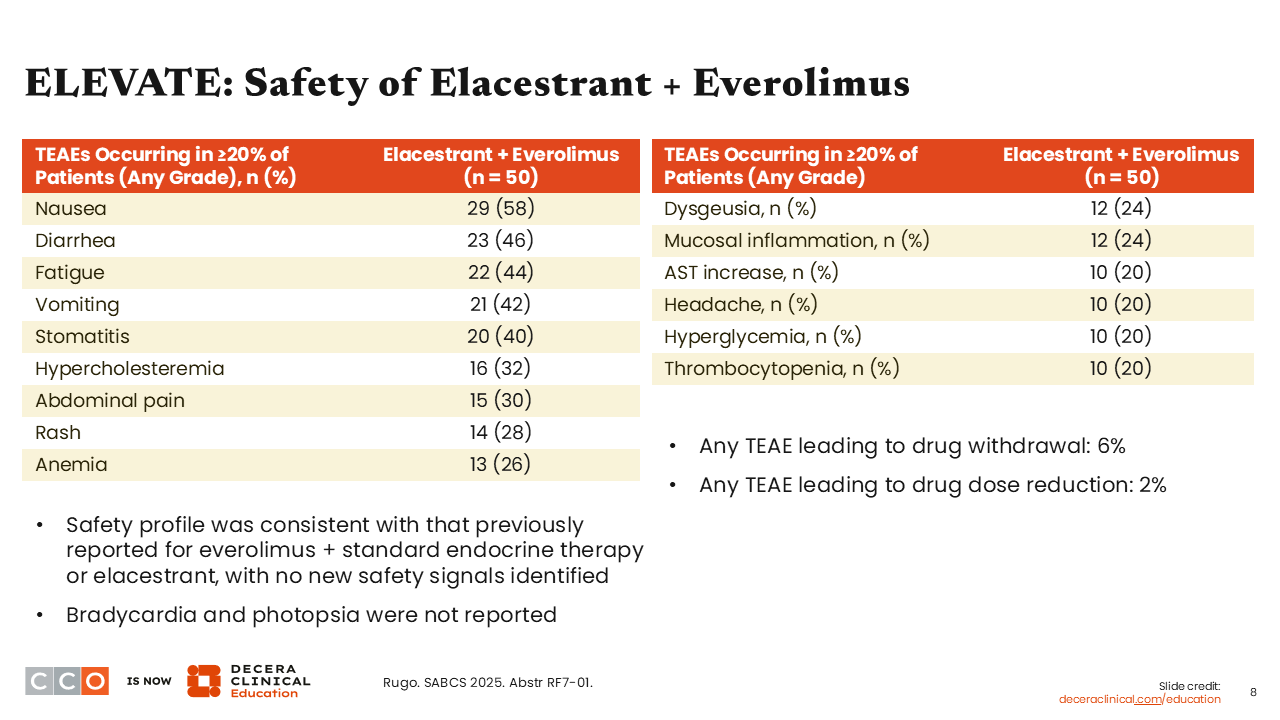
In the ELEVATE study, we can conclude that most of the toxicity presented with these combinations reflects the established safety profile of the targeted partner. The treatment-emergent adverse events (TEAEs) of any grade (in ≥20% of patients) in the everolimus arm are characteristic of everolimus’ safety profile, including nausea (58%), diarrhea (46%), and fatigue (44%). Investigators also reported stomatitis (40%) and rash (28%), which can be attributed to everolimus because of the known activity on the mTOR pathway.
Overall rates of TEAEs leading to patient withdrawal or dose reduction were low. Of note, no bradycardia or photopsia were reported— these are toxicities that I will discuss in further detail later in this activity.9
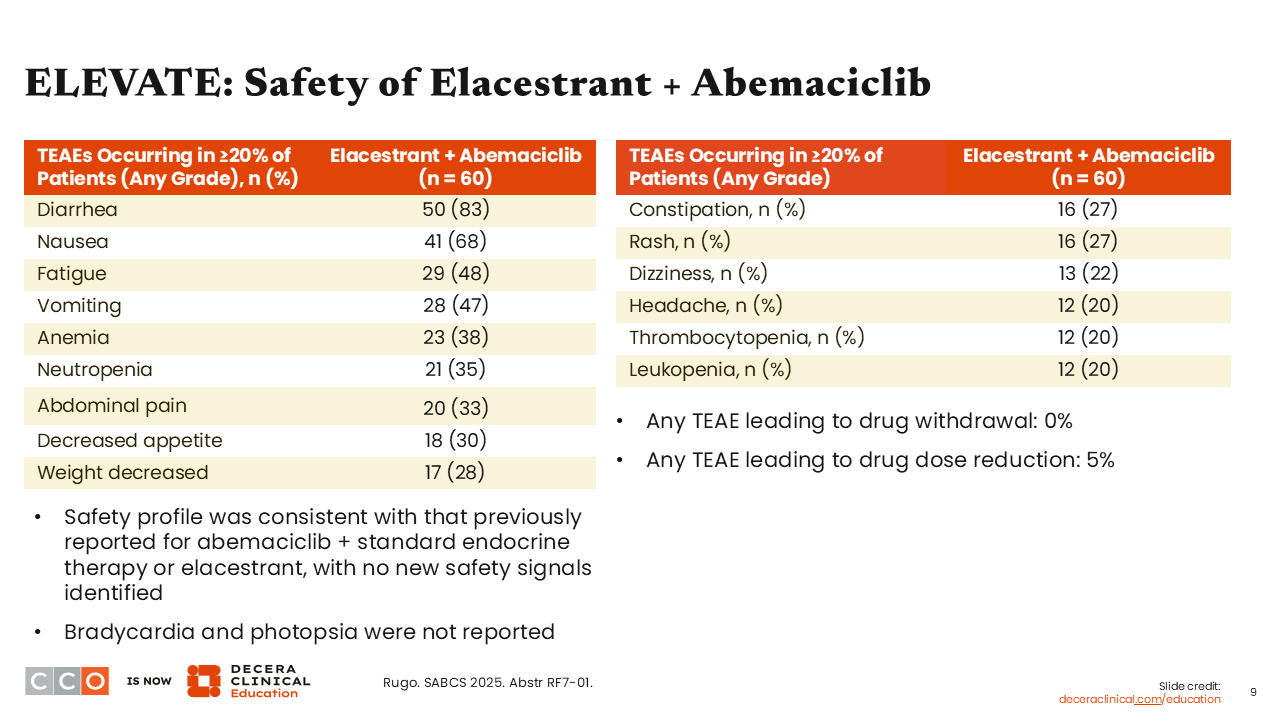
ELEVATE: Safety of Elacestrant + Abemaciclib
Erica L. Mayer, MD, MPH, FASCO:
Notable TEAEs of any grade (in ≥20% of patients) in the abemaciclib arm include diarrhea (83%), nausea (68%), and fatigue (48%). Investigators also reported anemia (38%) and neutropenia (35%), which are known adverse events (AEs) associated with abemaciclib. Here we see rates that are consistent for known AEs with abemaciclib.9
EMBER-3: Study Design
Erica L. Mayer, MD, MPH, FASCO:
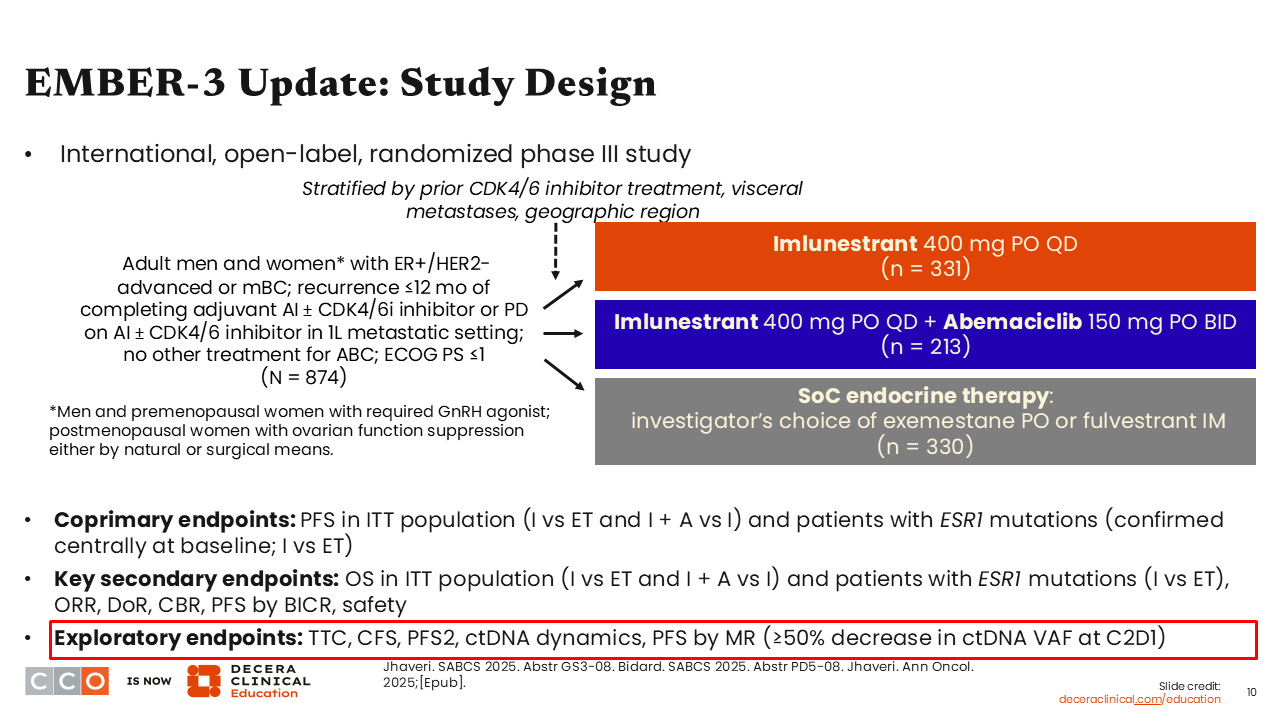
Let us look at another combination of an oral SERD with a targeted partner. This is an update from the phase III EMBER-3 trial which is evaluating imlunestrant with or without abemaciclib compared with SoC ET in patients with ER-positive/HER2-negative ABC and disease recurrence within 12 months of completing adjuvant therapy or disease progression in the first-line metastatic setting after treatment with an AI with or without a CDK4/6 inhibitor. No other prior treatments were allowed.
The trial had 2 primary endpoints. The first was PFS in the intention-to-treat (ITT) population for imlunestrant monotherapy vs SoC and imlunestrant monotherapy vs imlunestrant plus abemaciclib; the second was PFS in patients with ESR1 mutations for imlunestrant monotherapy vs SoC. Furthermore, the updated data presented at SABCS 2025 reported several secondary endpoints, including overall survival (OS), as well as exploratory endpoints that looked at PFS outcomes by molecular response (MR) in patients with ≥50% decrease in circulating tumor DNA (ctDNA) variant allele frequency (VAF) at C2D1.10,11
EMBER-3 Update: PFS (Primary Endpoint)
Erica L. Mayer, MD, MPH, FASCO:
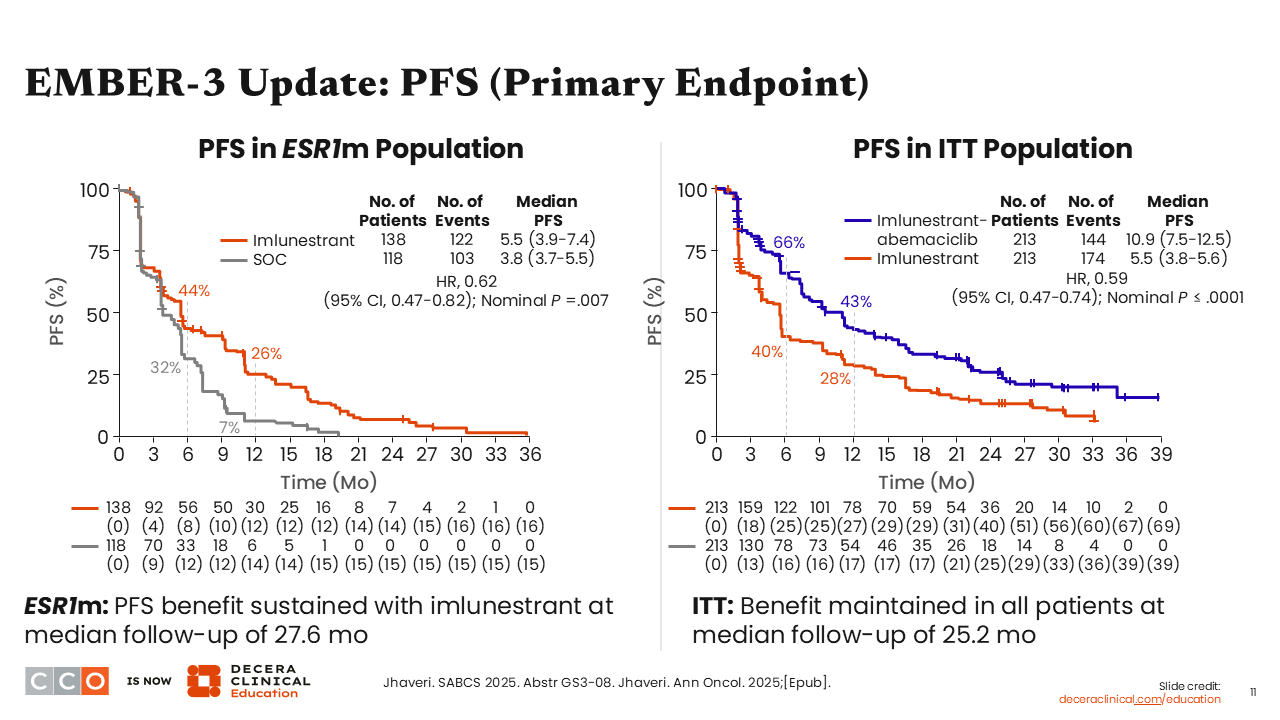
Looking at the updated PFS data for patients with an ESR1 mutation, those who received imlunestrant had a significant prolongation in PFS, with a median PFS of 5.5 months vs 3.8 months with SoC (HR: 0.62; 95% CI: 0.47-0.82; nominal P = .007). This is consistent with prior data, and these updated data led to the FDA approval of imlunestrant as monotherapy for adults with ER-positive, HER2-negative, ESR1 mutated ABC or MBC with disease progression after 1 or more prior lines of ET.12
We also saw updated data for PFS in the ITT population for the combination of imlunestrant plus abemaciclib vs imlunestrant monotherapy. Patients experienced almost a doubling in median PFS with imlunestrant plus abemaciclib (10.9 months) compared with imlunestrant monotherapy (5.5 months (HR: 0.59; 95% CI: 0.47-0.74; nominal P ≤.0001), which was consistent with prior data.10,13
EMBER-3: PFS by Subgroups I+A vs I
Erica L. Mayer, MD, MPH, FASCO:
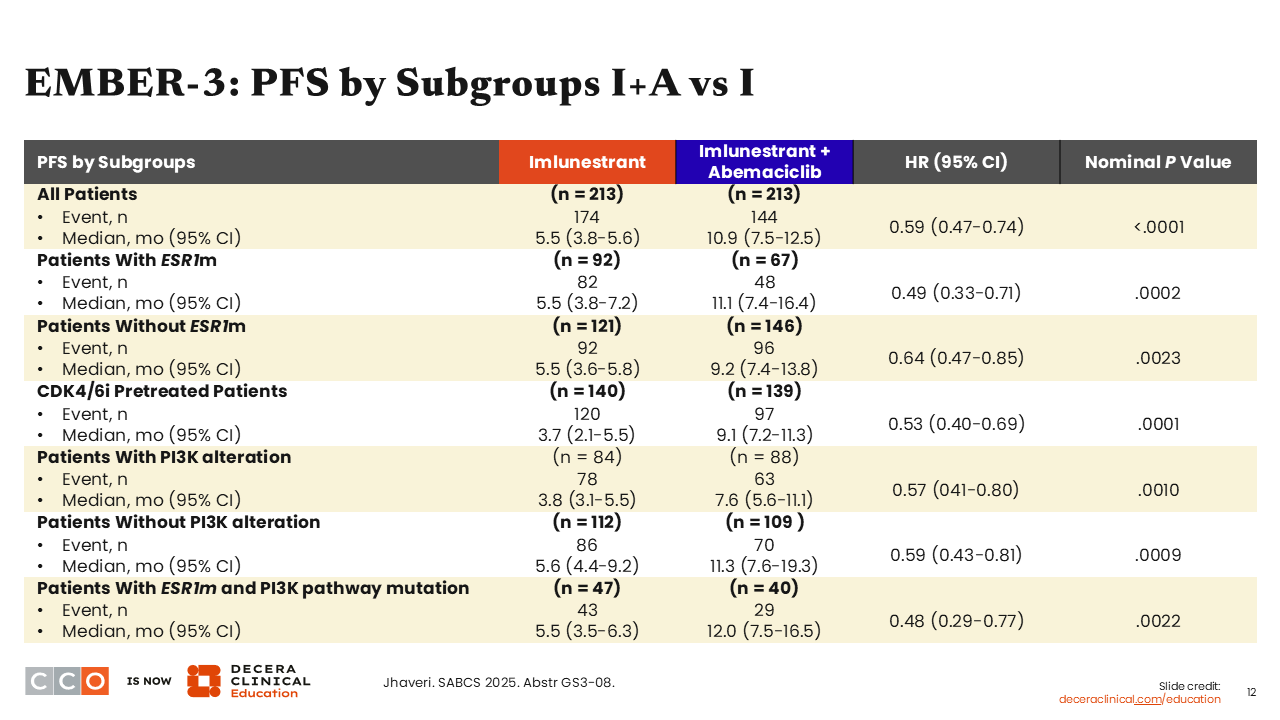
Looking at an analysis of patient subgroups, data for imlunestrant plus abemaciclib vs imlunestrant monotherapy show that the superior PFS benefit for the combination vs monotherapy was seen regardless of ESR1 mutation status. The median PFS for those with ESR1 mutations was 11.1 months with imlunestrant plus abemaciclib vs 5.5 months with imlunestrant monotherapy (HR: 0.49; 95% CI: 0.33-0.71; nominal P = .0002) and for those without an ESR1 mutation the median PFS was 9.2 months with imlunestrant plus abemaciclib vs 5.5 months with imlunestrant (HR: 0.64; 95% CI: 0.47-0.85); nominal P = .0023).
This suggests, as I have seen consistently in trials, that when a targeted partner is added to an oral SERD—meaning we are targeting the ER and a crosstalk resistance pathway—we can overcome any ESR1 mutation present in the tumor.
Other important subgroups include patients pretreated with a CDK4/6 inhibitor, where the PFS benefit was preserved. There also is benefit seen regardless of patients’ PIK3CA mutation status, particularly in patients with comutated ESR1 and PI3K pathway. Of note, that is a small patient population, making up approximately 20% of all patients in these cohorts. This is important because HCPs can be presented with a situation where they might wonder which therapy to use that targets a mutation in ESR1 or the PI3K pathway. Here, we see a preservation of benefit with a median PFS of 12.0 months with imlunestrant plus abemaciclib and of 5.5 months with imlunestrant monotherapy (HR: 0.48; 95% CI: 0.29-0.77; nominal P = .0022).10 This might suggest that it is reasonable and appropriate to target the ESR1 mutation with the use of an oral SERD in patients with mutations in both ESR1 and PIK3CA.
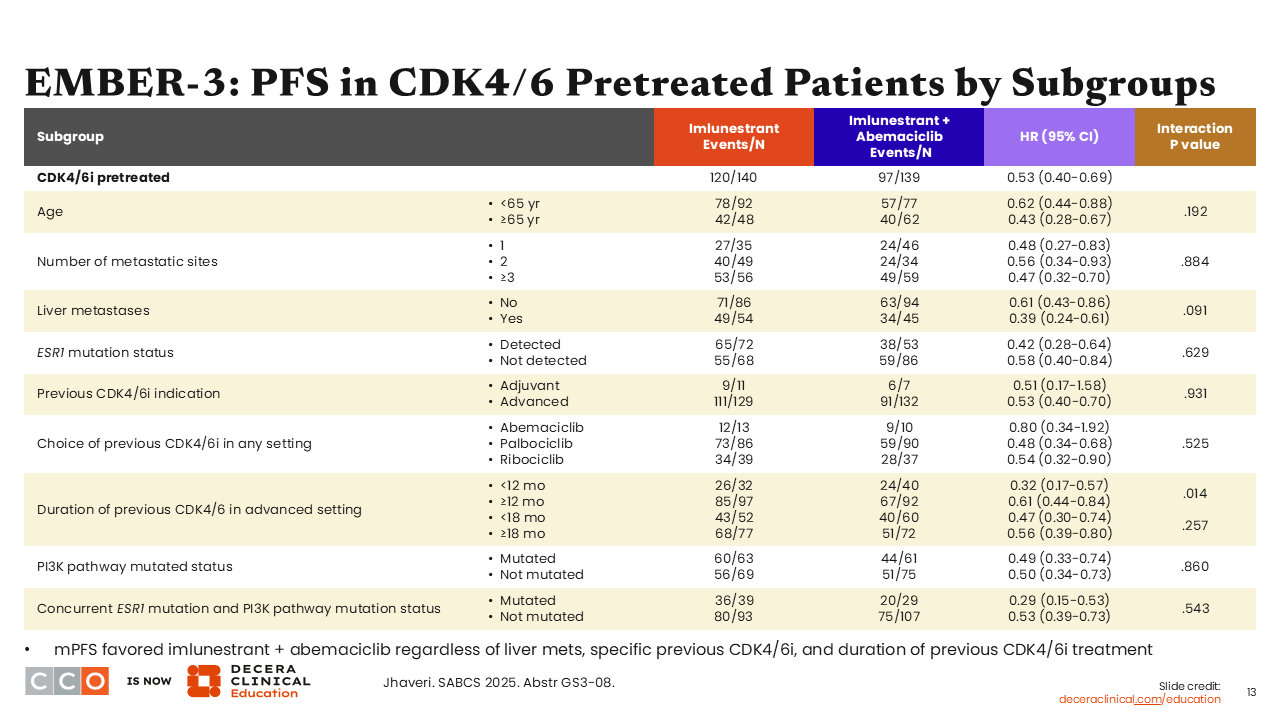
EMBER-3: PFS in CDK4/6 Pretreated Patients by Subgroups
Erica L. Mayer, MD, MPH, FASCO:
Consistent with previous data discussed earlier, median PFS analyses in the subgroups of patients who were previously treated with CDK4/6 inhibitors in the imlunestrant monotherapy and imlunestrant plus abemaciclib arms—approximately 70% to 86% of patients in these cohorts—show preservation of PFS benefit across all subgroups.10
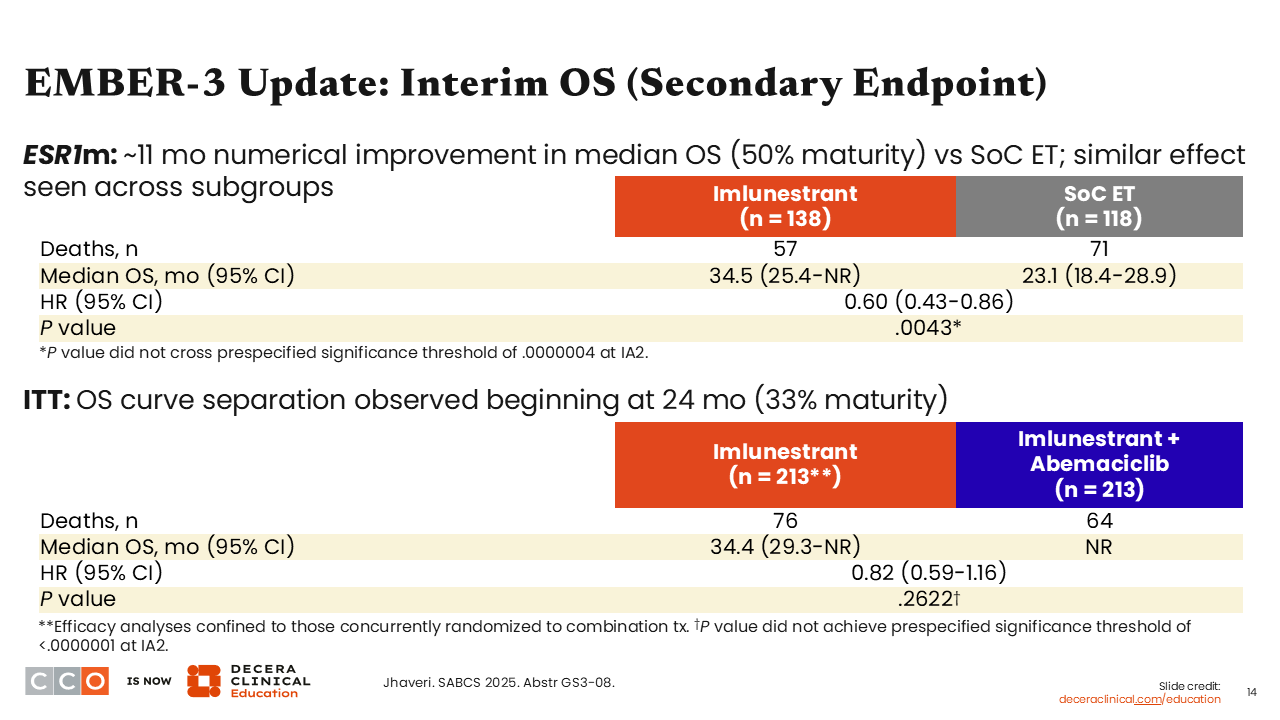
EMBER-3 Update: Interim OS (Secondary Endpoint)
Erica L. Mayer, MD, MPH, FASCO:
The EMBER-3 update also shared data for the secondary endpoint of OS with greater maturity compared with its previous report.14 For imlunestrant monotherapy vs SoC, the hazard ratio for OS was 0.60 (95% CI: 0.43-0.86; P = .0043). These data are not yet statistically significant but trending favorably at 50% maturity. In the comparison of imlunestrant plus abemaciclib vs imlunestrant monotherapy, the hazard ratio for OS was 0.82 (95% CI: 0.59-1.16; P = .2622). These data are at 33% maturity.10
When the initial data from EMBER-3 were reported,14 the OS data were at 15% maturity and the OS hazard ratio was trending in a somewhat unfavorable way, which was not surprising considering the maturity. Now, as more data are available, we are seeing a more favorable trend. Of course, we will need to continue monitoring this with longer follow-up of the trial.
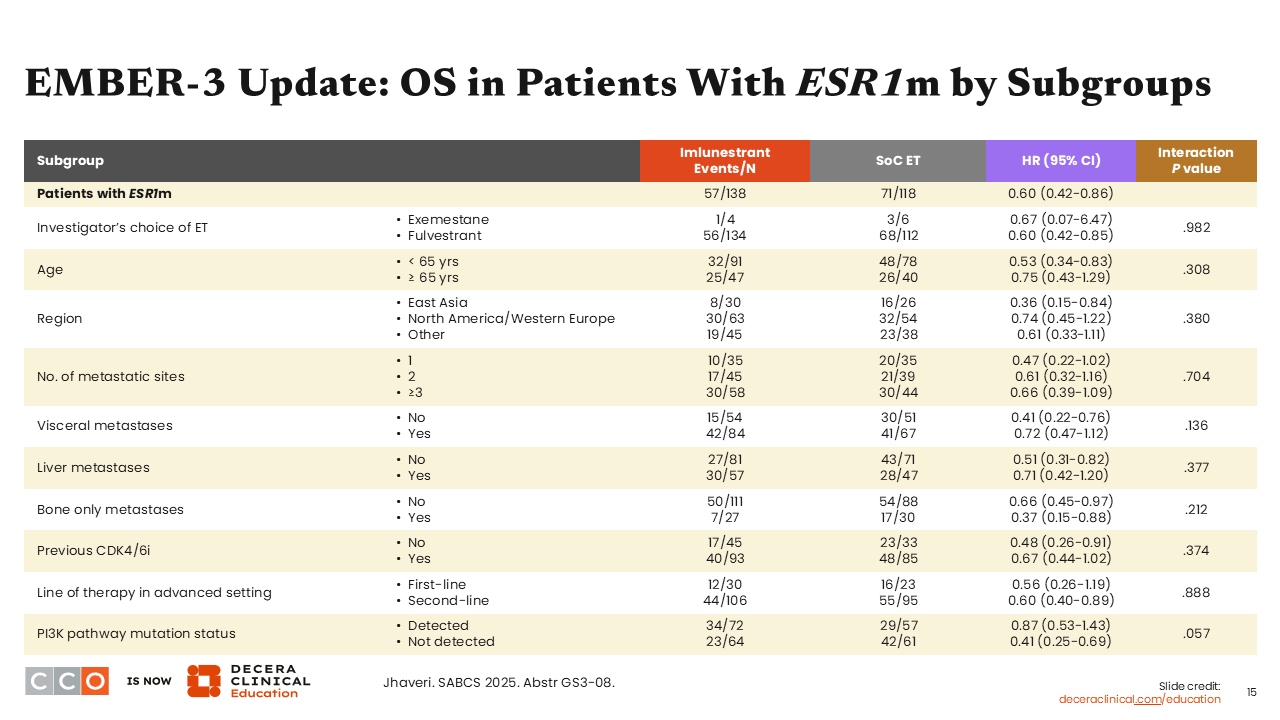
EMBER-3 Update: OS in Patients With ESR1m by Subgroups
Erica L. Mayer, MD, MPH, FASCO:
OS data across key patient subgroups, including with an ESR1 mutation, show a consistent trend of favorable hazard ratios for all prespecified subgroups.10
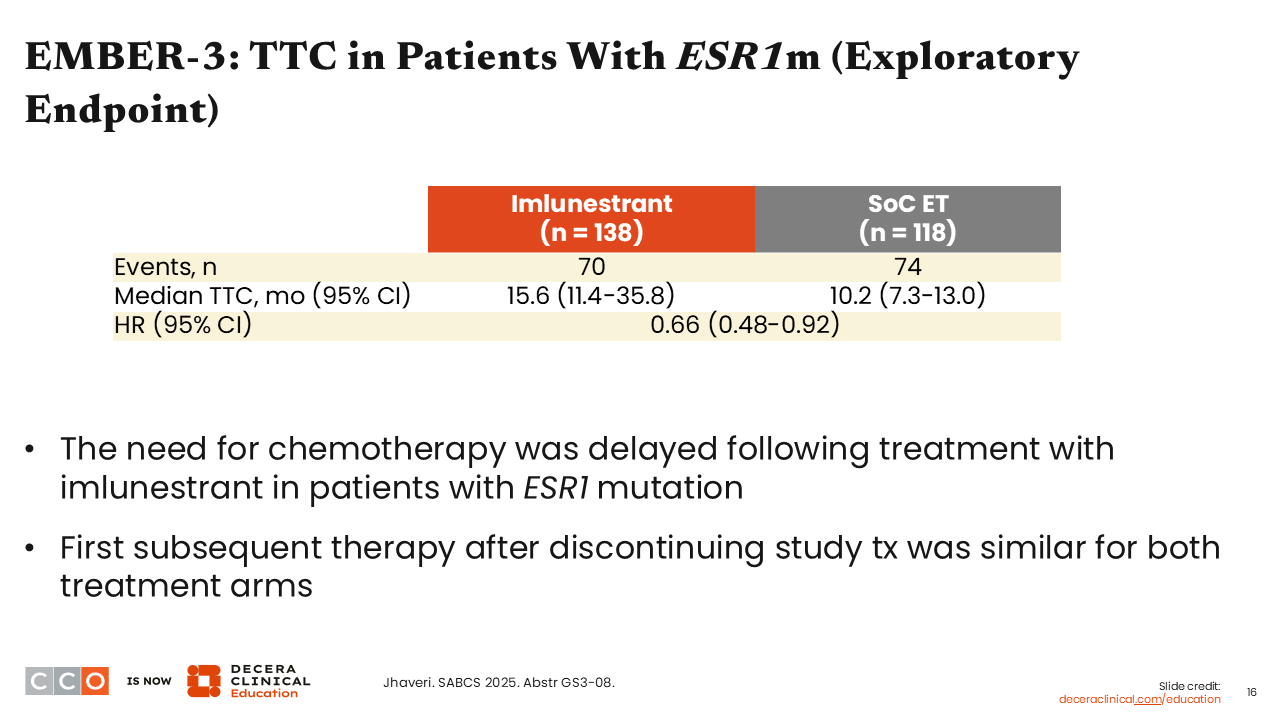
EMBER-3: TTC in Patients With ESR1m (Exploratory Endpoint)
Erica L. Mayer, MD, MPH, FASCO:
Regarding the exploratory endpoint of time to chemotherapy (TTC) in patients with an ESR1 mutation, we saw that TTC was delayed for those who received imlunestrant monotherapy with a median TTC of 15.6 months vs 10.2 months with SoC (HR: 0.66; 95% CI: 0.48-0.92), which is clinically meaningful.10
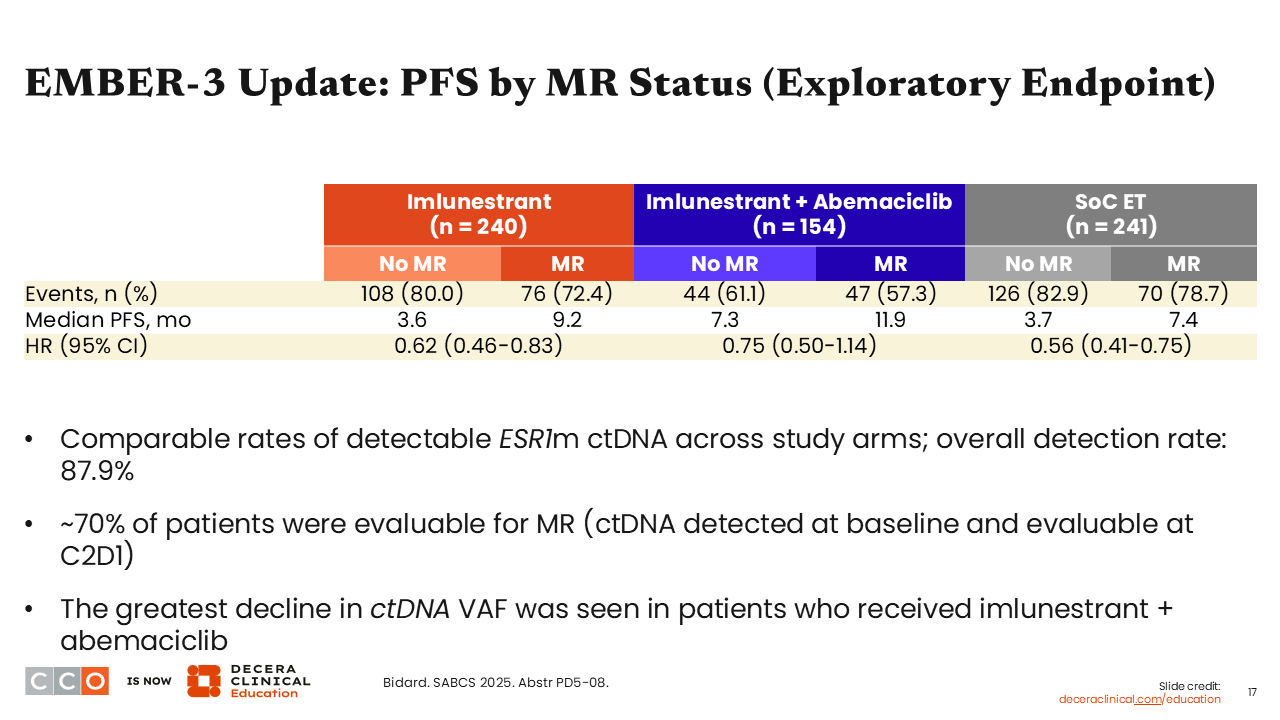
EMBER-3 Update: PFS by MR Status (Exploratory Endpoint)
Erica L. Mayer, MD, MPH, FASCO:
Another exploratory endpoint looked at PFS by MR status. ctDNA was measured at baseline and 4 weeks after treatment to evaluate the VAF for ESR1 mutation. Of note, MR was defined as having a 50% or greater reduction in ESR1 mutation VAF.
Looking at the changes in VAF, particularly in patients with a detected ESR1 mutation, there were profound reductions in detectable rates of ESR1-mutant ctDNA in patients who received imlunestrant alone or in combination with abemaciclib compared with those who received SoC alone. Furthermore, those who experienced MR achieved improved median PFS benefit vs those who did not experience an MR with imlunestrant alone (9.2 vs 3.6 months; HR: 0.62; 95% CI: 0.46-0.83), imlunestrant in combination with abemaciclib (11.9 vs 7.3 months; HR: 0.75; 95% CI: 0.50-1.14), and those who received SoC alone (7.4 vs 3.7 months; HR: 0.56; 95% CI: 0.41-0.75). These are exciting data because they suggest that, perhaps, there is a way for us to know who is going to do well on this therapy as soon as cycle 2, Day 1 of treatment. It begins to set up a pathway for us to get early readouts in the future to know whether we are going in the right direction with a specific treatment or if it is not going to be as favorable and we should change course. These data also are important from a pharmacodynamic point of view because they show us that the oral SERD is “hitting” the target.
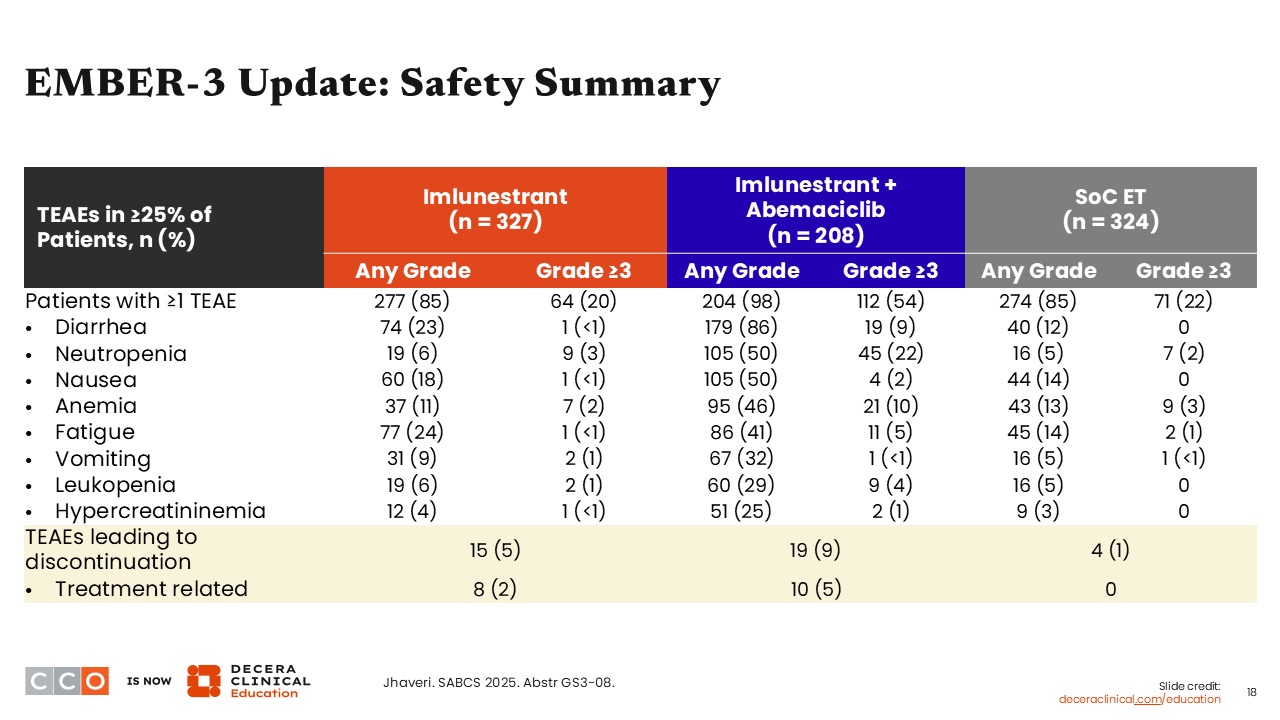
EMBER-3 Update: Safety Summary
Erica L. Mayer, MD, MPH, FASCO:
The safety data reported in the EMBER-3 update were consistent with what had been previously reported for this trial.10 As explained earlier, the toxicities reported are mostly characteristic of abemaciclib in the combination arm. Furthermore, imlunestrant monotherapy was extremely well tolerated with grade ≥3 neutropenia observed in 3% of patients and only a few grade ≥3 nausea (<1%), anemia (2%), fatigue (<1%), vomiting (1%) leukopenia (1%), and hypercreatininemia (<1%) in the monotherapy arm.10
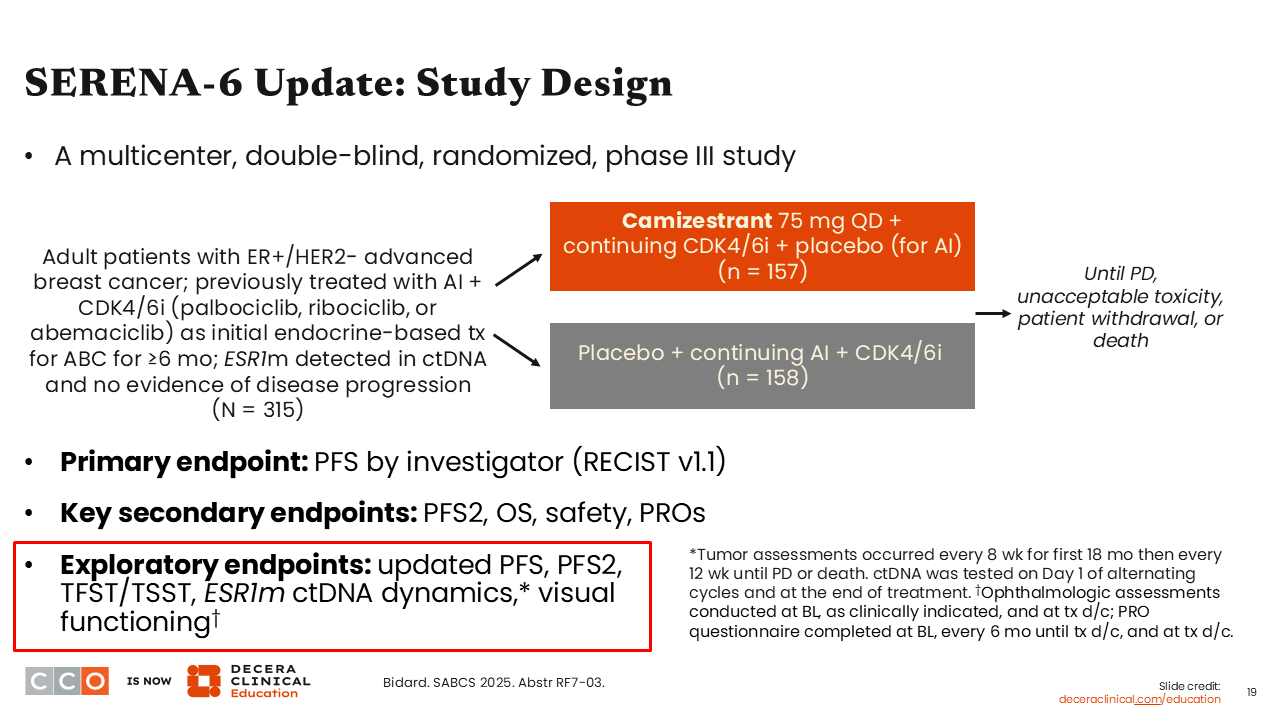
SERENA-6 Update: Study Design
Erica L. Mayer, MD, MPH, FASCO:
Moving onto another oral SERD combination trial, the phase III SERENA-6 trial. I call it a “1 and a half line study” because it was not strictly held in the first- or second-line setting. It lives in the middle. This was a 2-arm trial evaluating a switch to camizestrant plus a CDK4/6 inhibitor vs continuing an AI plus a CDK4/6 inhibitor in patients with HR-positive/HER2-negative ABC who were previously treated with an AI and CDK4/6 inhibitor and who after 6 months of treatment were determined (n = 315) to have an emerging ESR1 mutation that was detected prior to actual clinical progression on a scan.
The SERENA-6 primary endpoint of improving PFS by investigator per RECIST v1.1 was presented previously and reported to be positive in favor of a switch to camizestrant plus CDK4/6 inhibitor vs continuing AI plus CDK4/6 inhibitor (median PFS: 16.0 vs 9.2 months; HR: 0.44;95% CI: 0.31-0.60; P <.00001).15 The report at SABCS 2025 shared updated PFS data, as well as progression-free survival 2 (PFS2) and ESR1 mutation dynamics via ctDNA.16
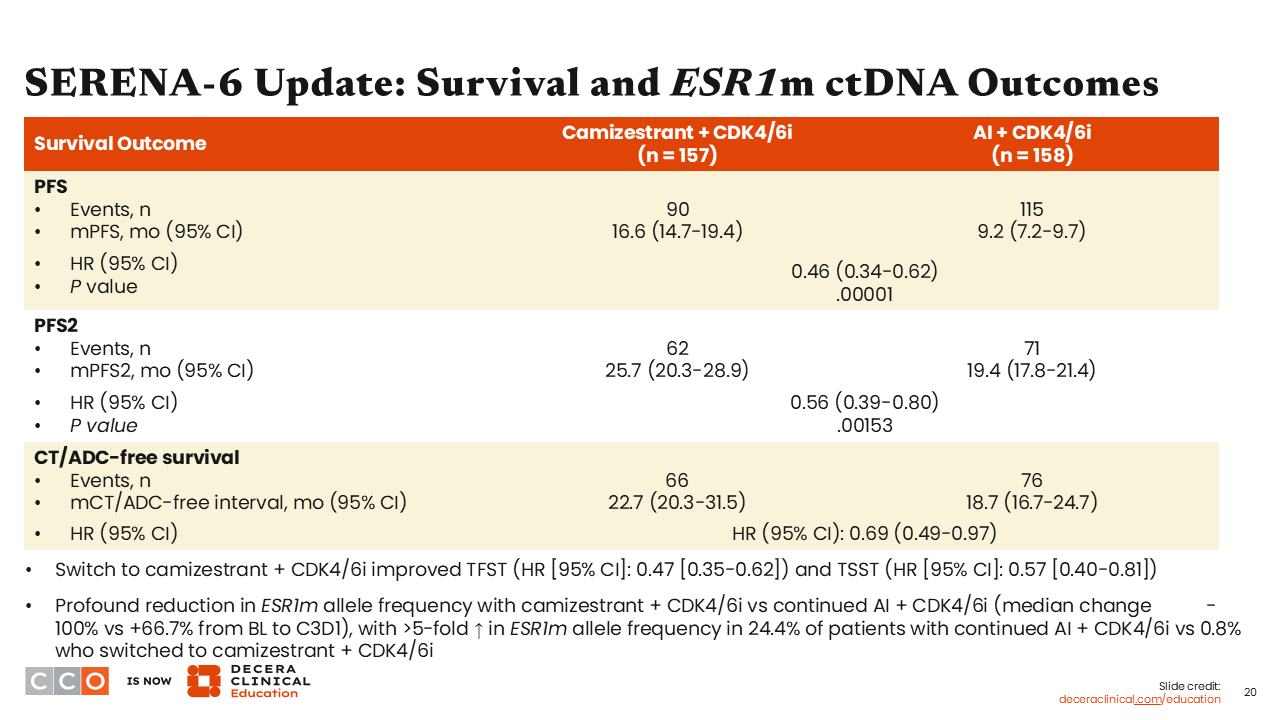
SERENA-6 Update: Survival and ESR1m ctDNA Outcomes
Erica L. Mayer, MD, MPH, FASCO:
In this update from SERENA-6, median PFS remains positive at 16.6 months in the camizestrant arm vs 9.2 months in the AI arm (HR: 0.46; 95% CI: 0.34-0.62; P = .00001). Median PFS2 was 25.7 in the camizestrant arm vs 19.4 months in the AI arm (HR: 0.56; 95% CI: 0.39-0.80; P = .00153). In addition, we also see improvement in chemotherapy or antibody–drug conjugate (ADC)–free survival both favoring the switch to camizestrant arm vs the AI continuation arm (HR: 0.69; 95% CI: 0.49-0.97), which is a clinically relevant endpoint.16
SERENA-6 Update: ESR1m ctDNA Dynamics
Erica L. Mayer, MD, MPH, FASCO:
Regarding results for ESR1 mutation ctDNA dynamics, in the camizestrant arm, the ESR1 mutation VAF decreased by a median 100% from baseline. By contrast, there was an increase by greater than 500% in the ESR1 mutation VAF for approximately 24% of patients in the AI continuation arm. These results indicate that an oral SERD is indeed targeting the ESR1 mutation on a molecular level when used prior to evidence of disease progression in imaging/scans.16
SERENA-6 Update: Summary of Visual Effects
Erica L. Mayer, MD, MPH, FASCO:
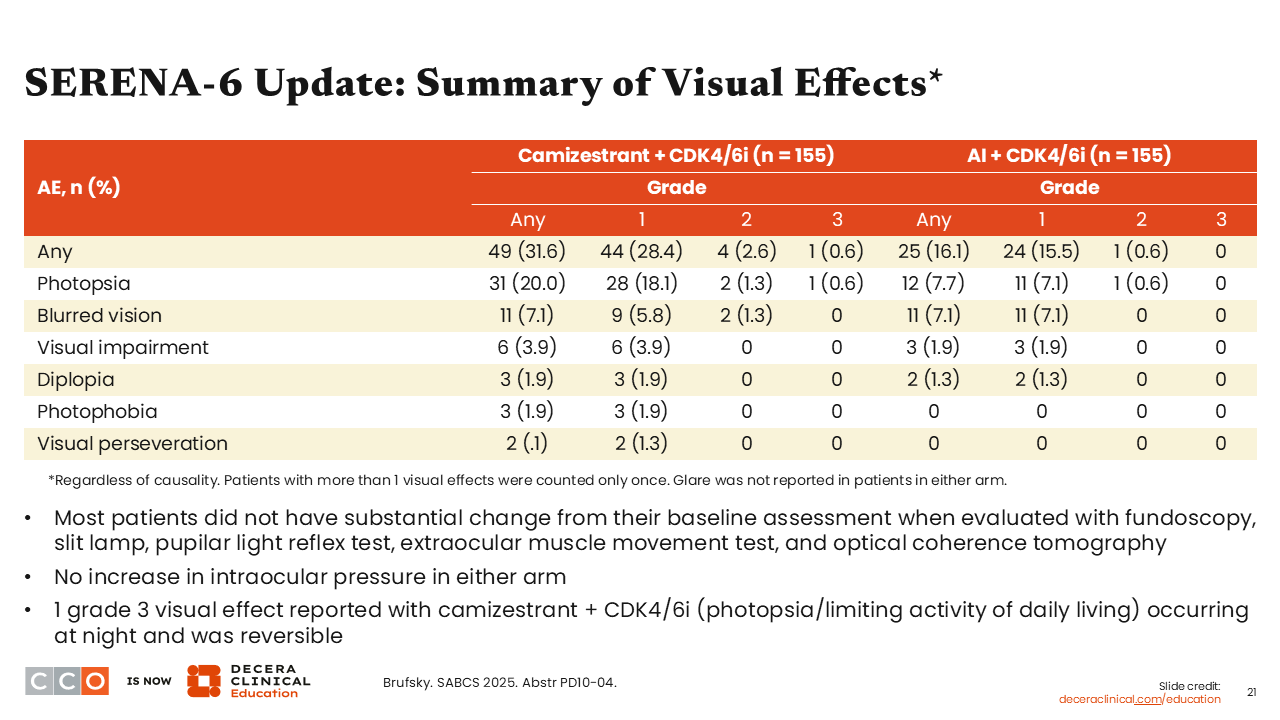
A second presentation on SERENA-6 looked specifically at the visual effects of camizestrant therapy. Camizestrant is an oral SERD that has a specific toxicity associated with it called photopsia, which means patients treated with this agent can see flashes, halos, sparks, streaks, or afterimages of light without directly looking at a light.17,18 This symptom is often brief and does not appear to be bothersome to patients. It does not appear to affect their daily functioning, but it is something that we would like to learn more about because it is a new toxicity for HCPs.
The analyses presented at SABCS 2025 reported visual effects with camizestrant in approximately 32% of patients in the camizestrant arm compared with only 16% of patients in the AI arm. This is important because patients are still experiencing ocular AEs with standard therapy. Furthermore, 7% of these patients experienced photopsia, which is not something HCPs normally think about with standard therapy, but it can happen. By contrast, 20% of patients experienced photopsia in the camizestrant arm.19 Most patients did not have substantial change from their baseline assessment when evaluated with fundoscopy, slit lamp, pupilar light reflex test, extraocular muscle movement test, and optical coherence tomography. Moreover, there was no increase in intraocular pressure in either arm. However, there was 1 case of grade 3 visual effect (photopsia) reported in the camizestrant arm limiting activity of daily living that occurred at night but was reversible.
SERENA 6 Update: Change From Baseline in Visual Acuity
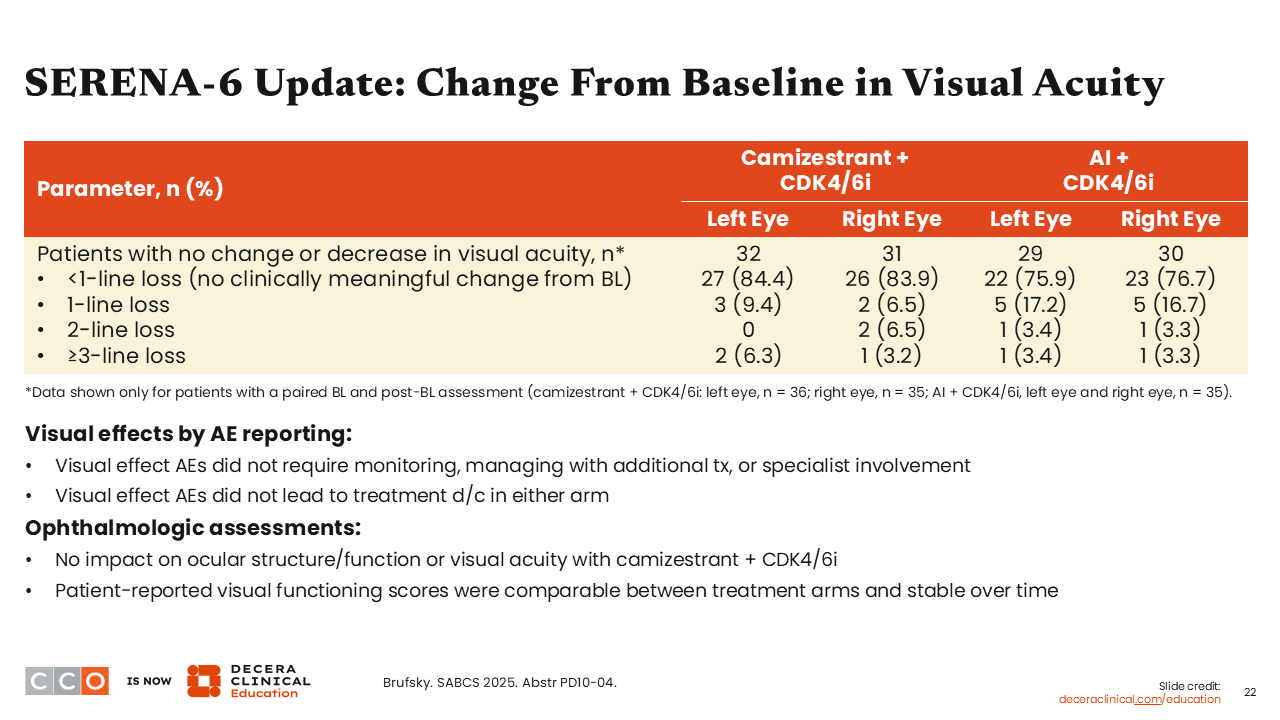
Erica L. Mayer, MD, MPH, FASCO:
Further analysis demonstrated no change in visual acuity among those in the camizestrant arm compared to the AI arm. We should note once more that photopsia has a quick onset; it is brief and generally does not impact patient’s activities.19
evERA: Study DESIGN
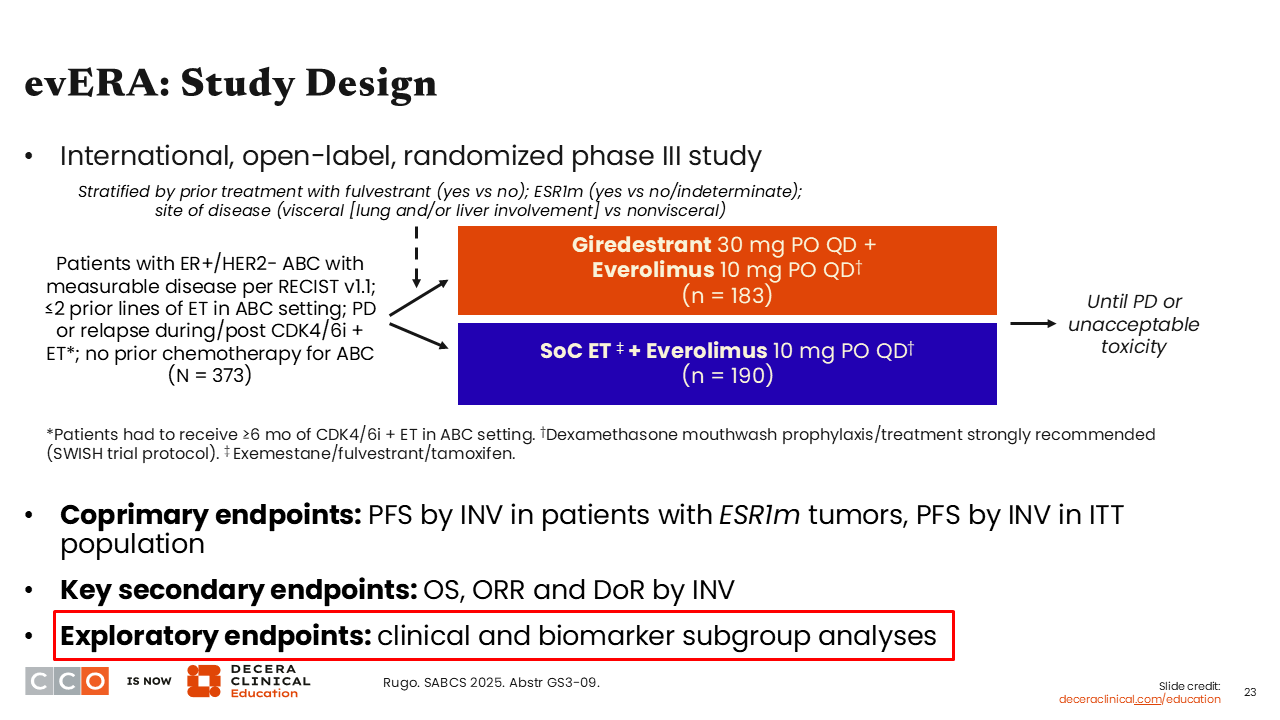
Erica L. Mayer, MD, MPH, FASCO:
Now I want to discuss the update presented for the phase III evERA trial evaluating the oral SERD giredestrant plus everolimus vs SoC ET plus everolimus in patients with ER-positive, HER2-negative ABC previously treated with a CDK4/6 inhibitor and no prior treatment with chemotherapy (n = 373). The primary endpoint from evERA was previously reported showing giredestrant plus everolimus improved PFS by investigator in patients with ESR1 mutation (median PFS: 9.99 vs 5.45 months; HR: 0.38; 95% CI: 0.27-0.54; P <.0001) and in ITT (median PFS: 8.77 vs 5.49 months; HR: 0.56; 95% CI: 0.44-0.71; P <.0001).20
Updated data presented clinical and biomarker subgroup analyses.21
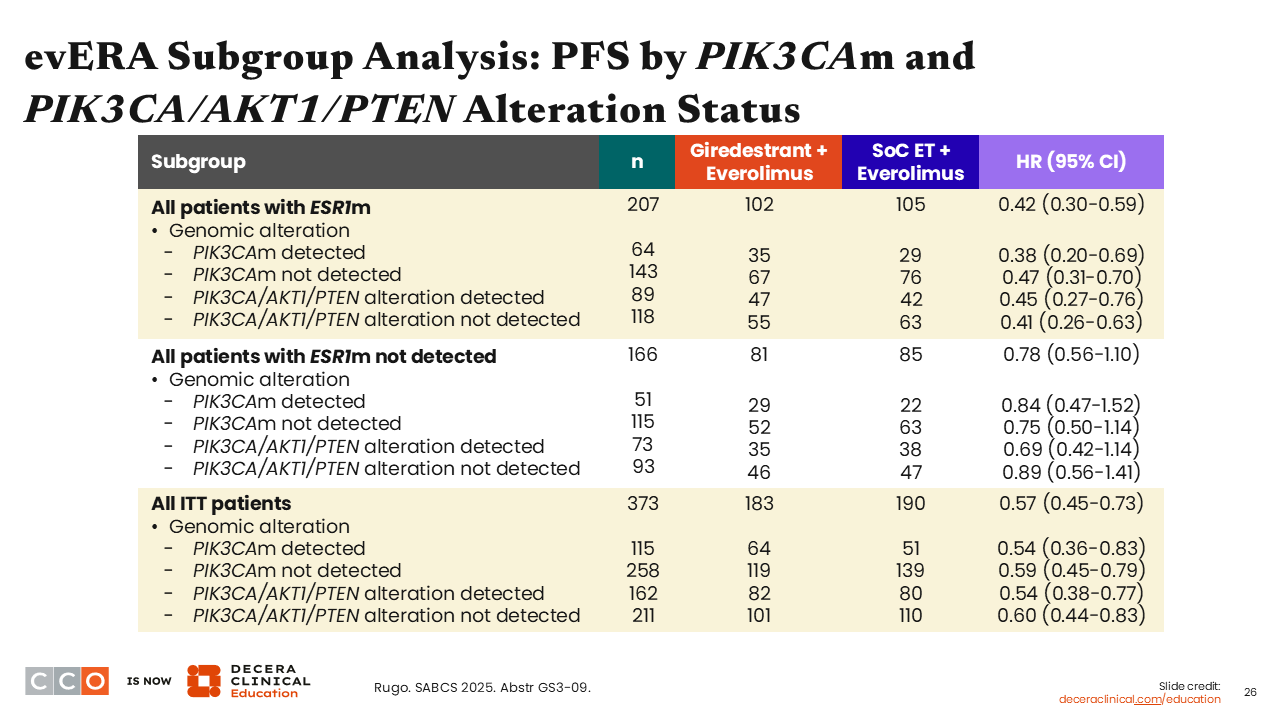
evERA Subgroup Analysis: PFS in Subgroups
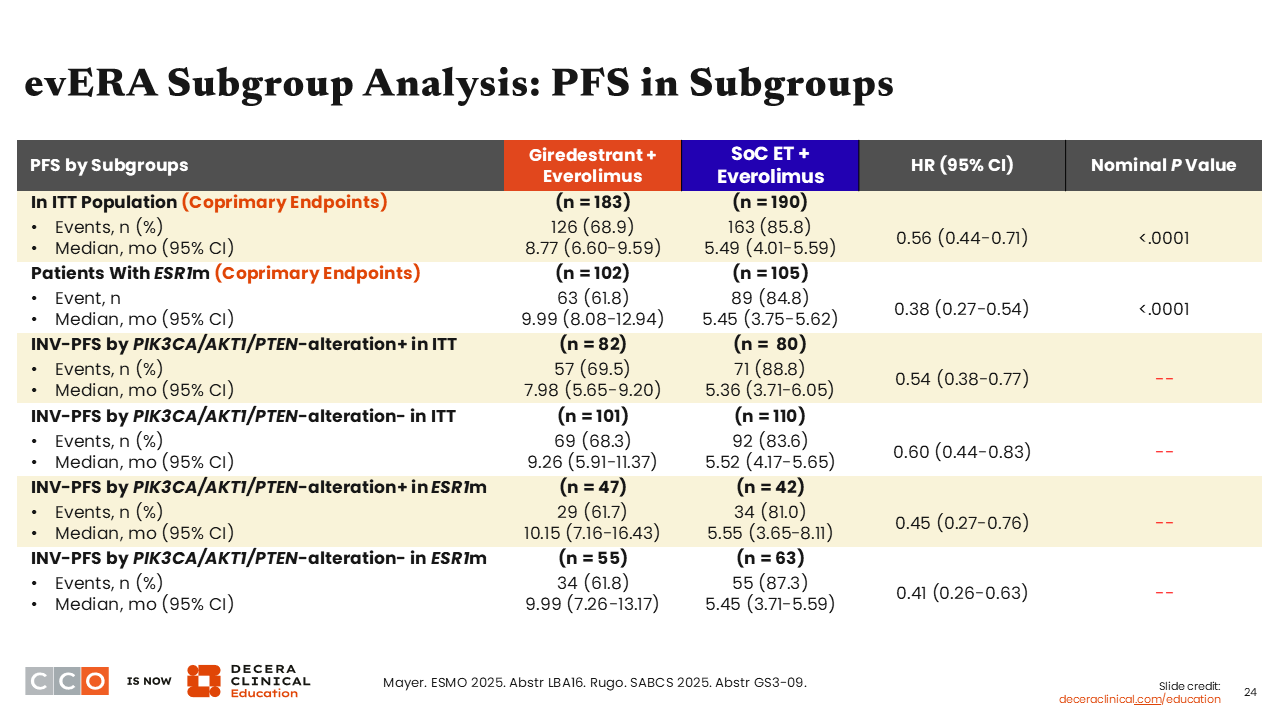
Erica L. Mayer, MD, MPH, FASCO:
When looking at PFS in subgroups assessed by investigators in patients with PIK3CA/AKT1/PTEN alteration in the overall population, there was consistent benefit in favor of giredestrant plus everolimus vs SoC ET plus everolimus (HR: 0.54; 95% CI: 0.38-0.77). Similarly, benefit was consistent whether the patient was ESR1 mutated together with a PIK3CA/AKT1/PTEN alteration (HR: 0.45 (95% CI: 0.27-0.76) or without PIK3CA/AKT1/PTEN alteration (HR: 0.41; 95% CI: 0.26-0.63).
Although not shown here, we also saw a favorable trend in interim OS with giredestrant plus everolimus vs SoC ET plus everolimus in patients without an ESR1 mutation.20
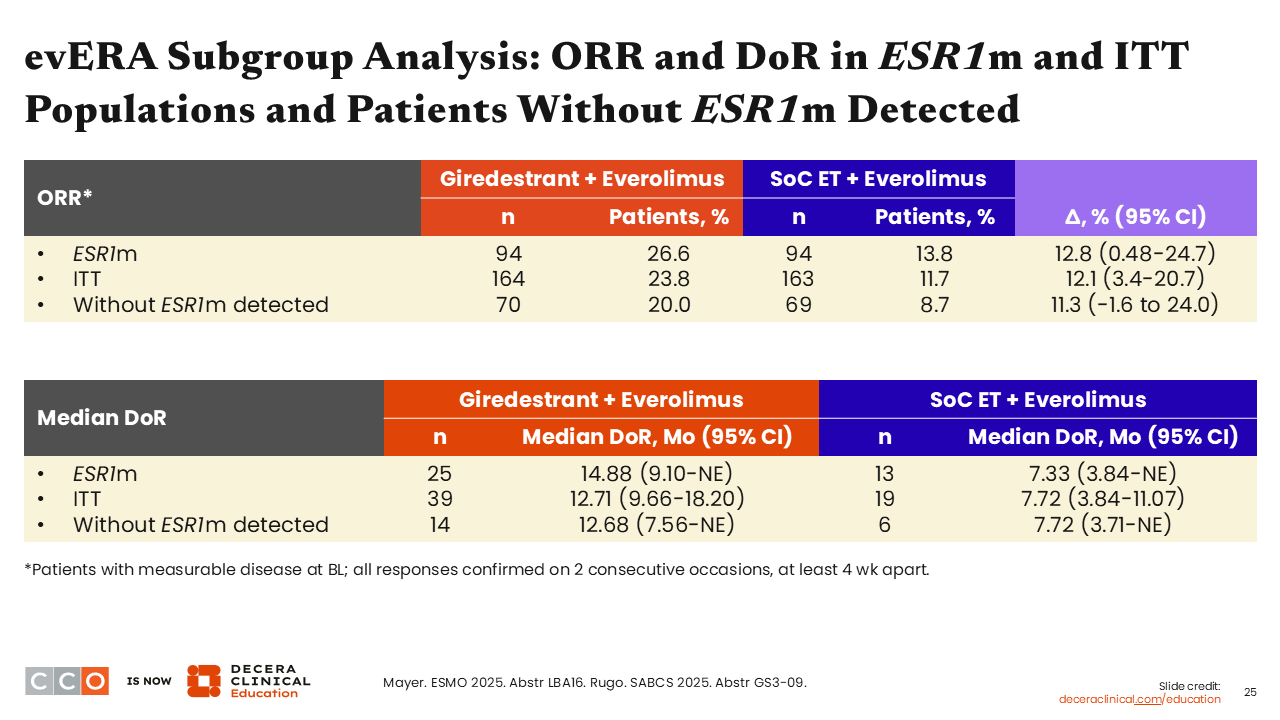
evERA Subgroup Analysis: ORR and DoR in ESR1m and ITT Populations and Patients Without ESR1m Detected
Erica L. Mayer, MD, MPH, FASCO:
ORRs were essentially doubled in the giredestrant plus everolimus arm vs the SoC ET plus everolimus arm in all patient subgroups—ESR1 mutation present (26.6% vs 13.8%), ITT (23.8% vs 11.7%), and no ESR1 mutation (20.0% vs 8.7%). In addition, median DoRs were increased with the use of giredestrant plus everolimus compared with SoC ET plus everolimus in patients with an ESR1 mutation (14.88 vs 7.33 months), in the ITT population (12.71 vs 7.72 months), and in patients without an ESR1 mutation (12.68 vs 7.72 months).21
evERA Subgroup Analysis: PFS by PIK3CAm and PIK3CA/AKT1/PTEN Alteration Status
Erica L. Mayer, MD, MPH, FASCO:
Looking at these data in another way, you can see that the PFS benefit is significant in patients with an ESR1 mutation and the overall ITT population. Both are the key patient populations for the trial’s primary endpoint. Although the PFS benefit in those without an ESR1 mutation is trending also in favor of the giredestrant arm, it is not significant in this setting.21
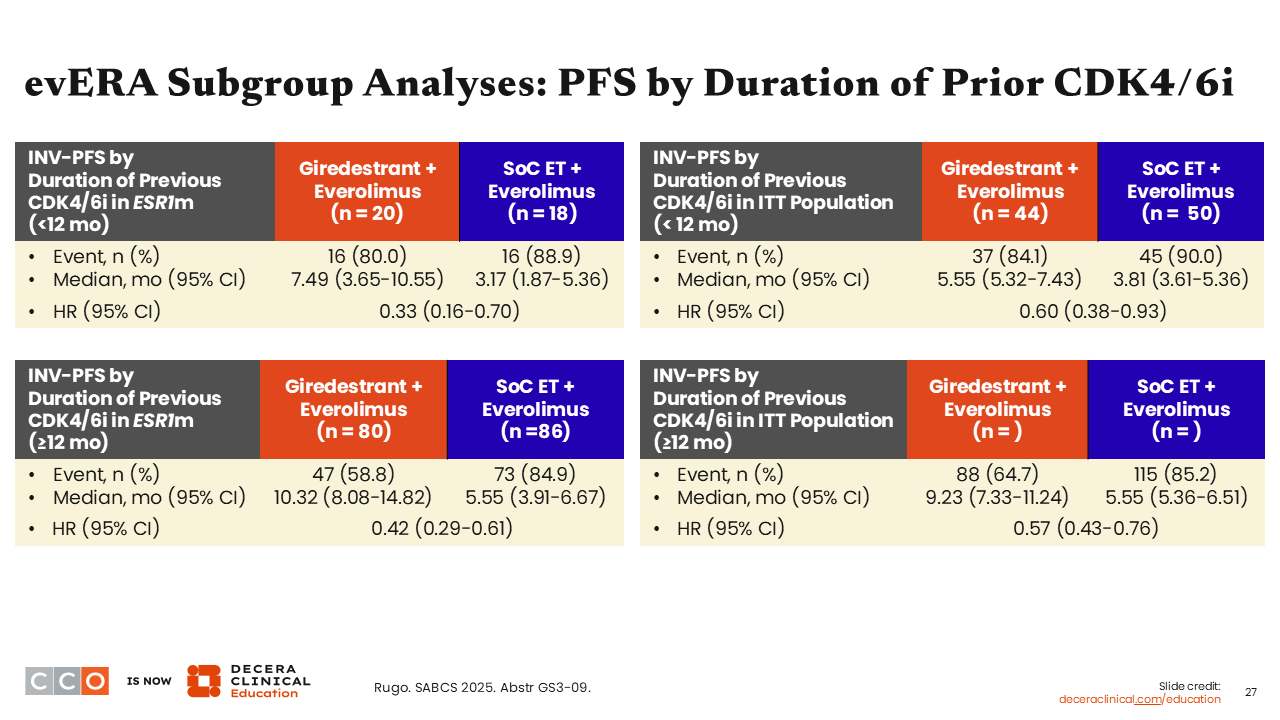
evERA Subgroup Analyses: PFS by Duration of Prior CDK4/6i
Erica L. Mayer, MD, MPH, FASCO:
In addition, consistent PFS benefit was seen regardless of patients’ duration on prior CDK4/6 inhibitor therapy. In patients with ESR1-mutant disease who received CDK4/6 inhibitors for either less than 12 months (HR: 0.33; 95% CI: 0.16-0.70) or 12 months or more (HR: 0.42; 95% CI: 0.29-0.61), a favorable trend was observed for giredestrant with <12 months on CDK4/6 inhibitor having a slightly lower hazard ratio than the ≥12 months duration.21 This trend was sustained but was less robust in the ITT population who received CDK4/6 inhibitors for either less than 12 months (HR: 0.60; 95% CI: 0.38-0.93) or 12 months or more (HR: 0.57; 95% CI: 0.43-0.76).
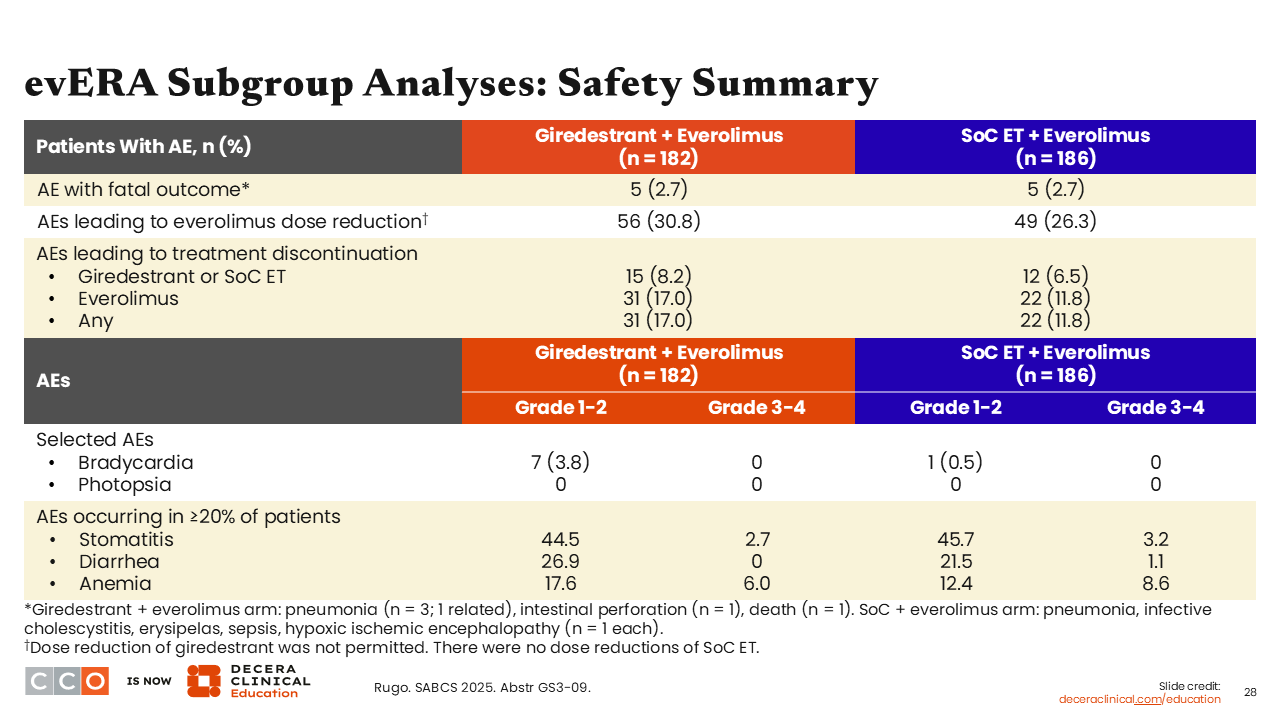
evERA Subgroup Analyses: Safety Summary
Erica L. Mayer, MD, MPH, FASCO:
Safety data were stable and consistent with prior reports.20 In general, the safety profile of giredestrant plus everolimus was primarily driven by everolimus, so there is little toxicity contributed by giredestrant itself.
Furthermore, treatment discontinuation rates were low: 8.2% in the giredestrant plus everolimus arm vs 6.5% in the SoC ET plus everolimus arm. Grade 1/2 bradycardia was reported in both arms (3.8% with giredestrant and 0.5% with SoC ET), which is seen with some oral SERDs, but these were generally asymptomatic and did not require intervention. No photopsia AEs were reported.21
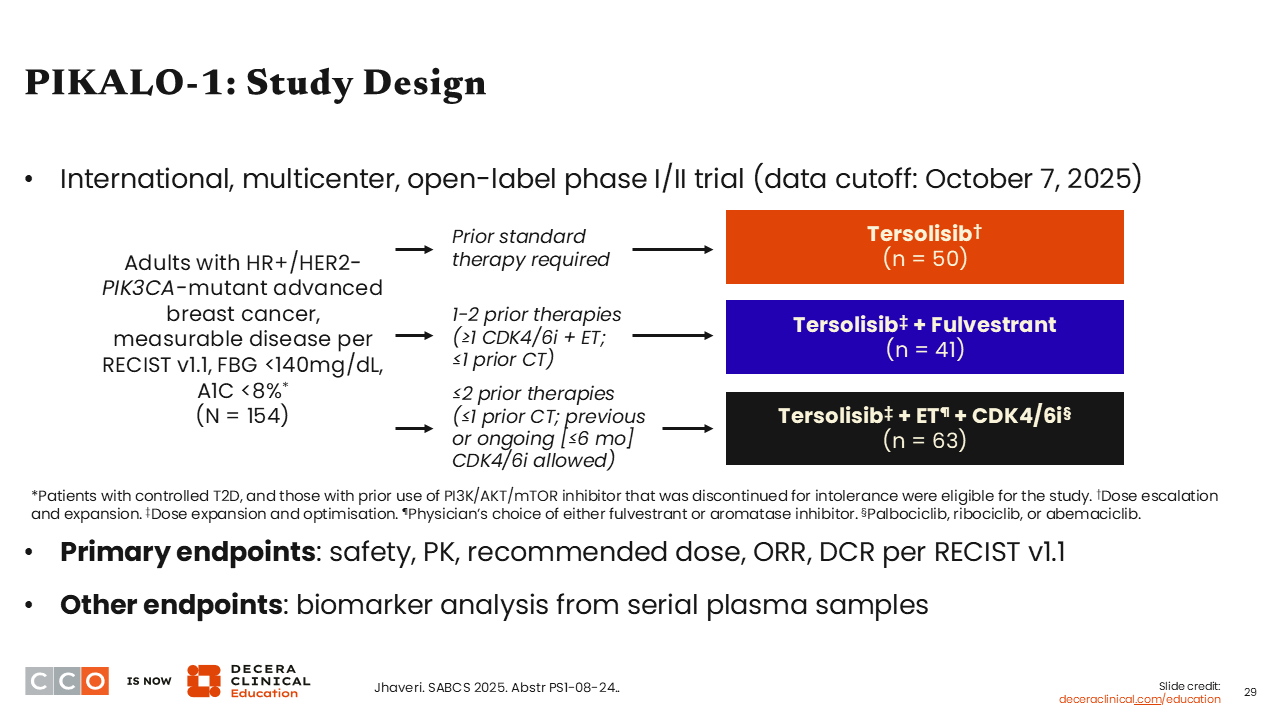
PIKALO-1: Study Design
Erica L. Mayer, MD, MPH, FASCO:
Now I am going to move into some of the emerging data for novel PI3K inhibitors. Data from the phase I/II PIKALO-1 trial were presented at SABCS 2025. This was a study of the novel oral central nervous system (CNS)–penetrating PI3Kα inhibitor tersolisib, which specifically targets the PIK3CA H1047X helical- and kinase-domain mutations (eg, H1047R). In addition to oral SERDs, this is an important category of agents because while they target constitutively active PI3K protein in tumor cells, it might theoretically spare PI3K in liver as well as in muscle cells.22,23 This helps reduce the toxicities like hyperglycemia that are often seen with traditional PI3K inhibitors. Of note, hyperglycemia is a challenging toxicity to manage in clinic.
The 3-arm PIKALO-1 trial evaluated tersolisib alone vs in combination fulvestrant or either fulvestrant or AI plus a CDK4/6 inhibitor in patients with HR-positive/ HER2-negative, PIK3CA-mutated ABC (N = 154). The primary endpoints were safety, pharmacokinetics, recommended phase II dose, and DCR per RECIST v1.1. Enrolled patients included those with previously treated disease, fasting blood glucose <140mg/dL and an A1C less than 8%.24
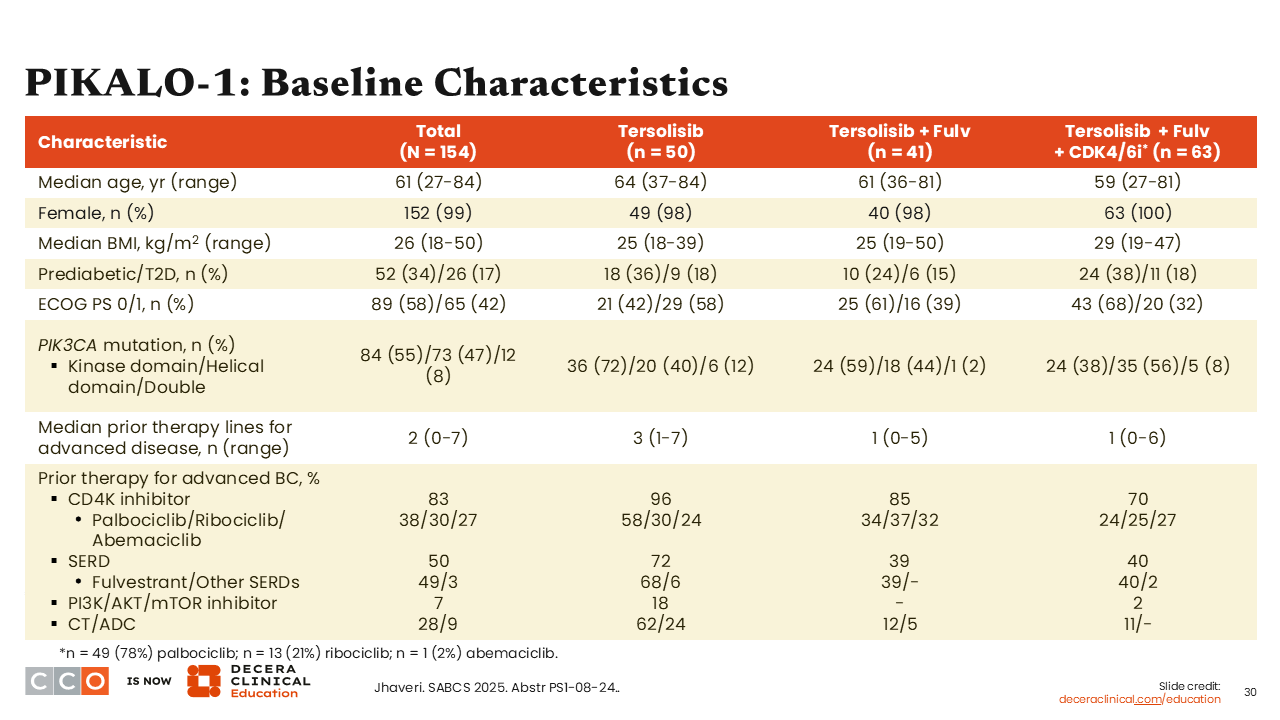
PIKALO-1: Baseline Characteristics
Erica L. Mayer, MD, MPH, FASCO:
When we look at the baseline characteristics of patients who participated in this study, there were a notable proportion who would be considered in prediabetes (34%) or type 2 diabetes (17%), and there was a median of 2 prior lines of therapy (range: 0-7). Also important to note is that approximately 7% of patients previously received a PI3K/AKT/mTOR inhibitor.24
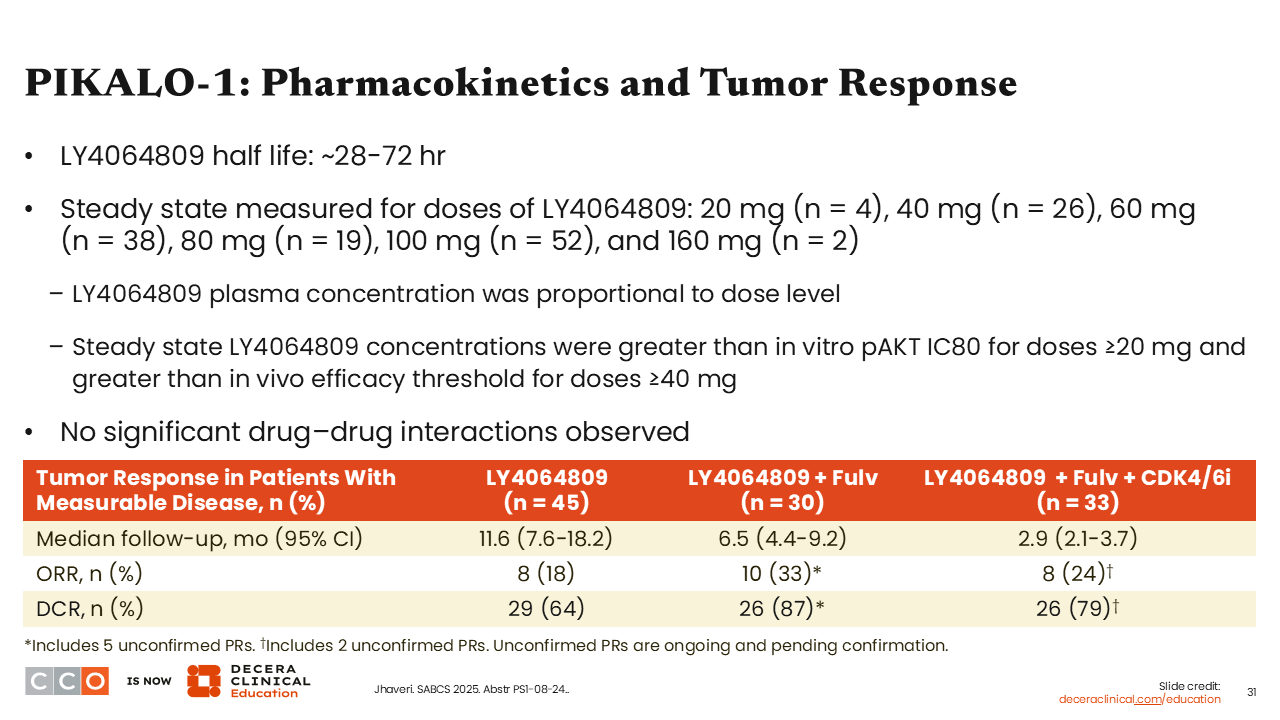
PIKALO-1: Pharmacokinetics and Tumor Response
Erica L. Mayer, MD, MPH, FASCO:
Focusing on pharmacokinetics and tumor response, we saw that tersolisib had a half-life ranging from 28-72 hours, plasma concentration was proportional to dose, and steady state concentrations of tersolisib were above the in vitro phosphorylated AKT IC80 at doses ≥20 mg and greater than the in vivo efficacy threshold for doses ≥40 mg.
In these preliminary analyses we saw that patients who received tersolisib alone achieved an ORR of 18% (n = 8) and DCR of 64% (n = 29), whereas patients receiving tersolisib plus fulvestrant had an ORR of 33% (n = 10) and DCR of 87% (n = 26). Finally, those who received tersolisib plus fulvestrant and a CDK4/6 inhibitor achieved an ORR of 24% (n = 24) and DCR of 79% (n = 26). I think these data are encouraging and suggest tersolisib is an active agent in this population of patients with PI3K mutations.24
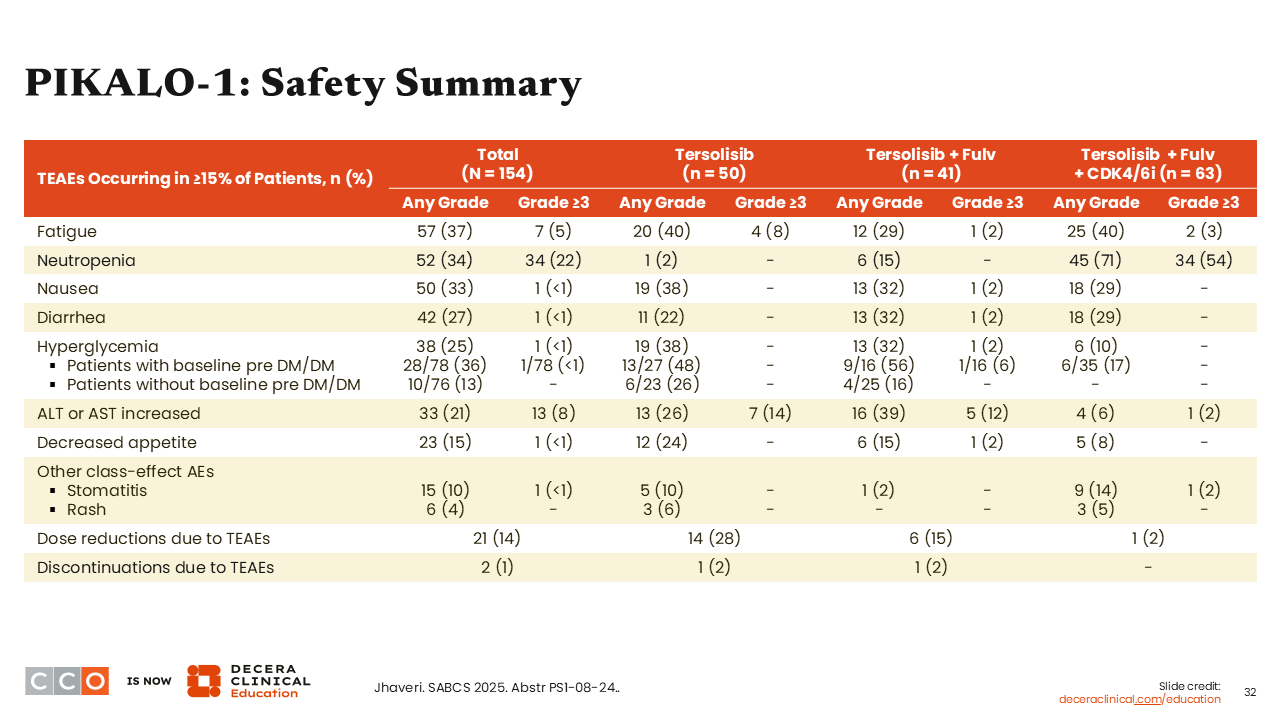
PIKALO-1: Safety Summary
Erica L. Mayer, MD, MPH, FASCO:
Regarding safety, I want to focus on hyperglycemia. Rates of any-grade hyperglycemia were approximately 25% for all patients, and there were minimal grade 3 or higher hyperglycemia AEs—which was specifically seen in 1 patient with prediabetes or type 2 diabetes at baseline. This is encouraging considering what we have previously seen with other PI3K inhibitors. Historically, PI3K inhibitors are known to be associated with hyperglycemia including in patients selected for lower risk of developing hyperglycemia than what was enrolled in this trial.
Overall, there were relatively low rates of diarrhea (any grade: 27%; grade ≥3: <1%) and rash (any grade: 4%; grade ≥3: 0.0%).24
PIKALO-1: Impact on Glucose Metabolism and ctDNA
Erica L. Mayer, MD, MPH, FASCO:
A deeper dive into the reported AEs of hyperglycemia showed that there was minimal impact on mean fasting glucose for patients with or without diabetes. Furthermore, there was a decrease in PIK3CA-mutant ctDNA VAF at cycle 2, which is another favorable endpoint to identify patients who are favorably responding to targeted therapy. Given the promising data from this phase I/II study, a phase III study evaluating tersolisib together with other targeted agents is now open for enrollment (NCT07174336).
So stay tuned for more data on tersolisib, as it is entering a phase III trial that will investigate the triplet therapy of tersolisib plus ET and a CDK4/6 inhibitor. I encourage you to support this trial, too, because this may be the next new thing for patients with ABC and PI3KCA mutations.24