CME
Key Studies in Lung Cancer: Independent Conference Coverage of ESMO 2025
European Learners: 1.50 EBAC® CE Credit
Physicians: Maximum of 1.50 AMA PRA Category 1 Credits™
Released: December 16, 2025
Expiration: June 15, 2026
Activity
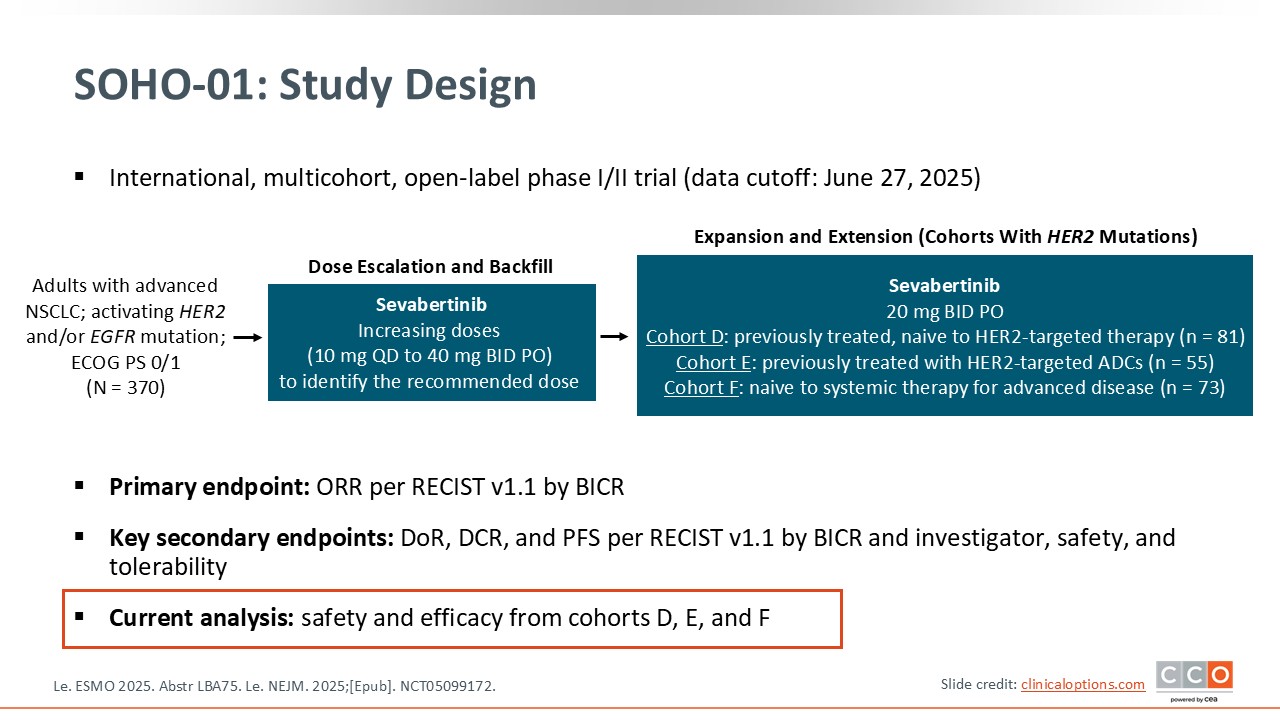
SOHO-01: Phase I/II Study of Sevabertinib in Advanced HER2-Mutant NSCLC
David Planchard, MD, PhD:
The SOHO-01 trial evaluated sevabertinib in a nonrandomized, phase II setting with the aim of assessing both efficacy and safety in patients with advanced NSCLC and activating HER2 mutations.8 The study enrolled both previously treated and treatment-naive patients. The primary endpoint was response rate, analyzed across several predefined cohorts.
The trial consisted of 3 main groups: treatment-naive patients, patients who had previously received HER2-targeted ADCs, and patients who were previously treated but remained naive to HER2-directed therapies.
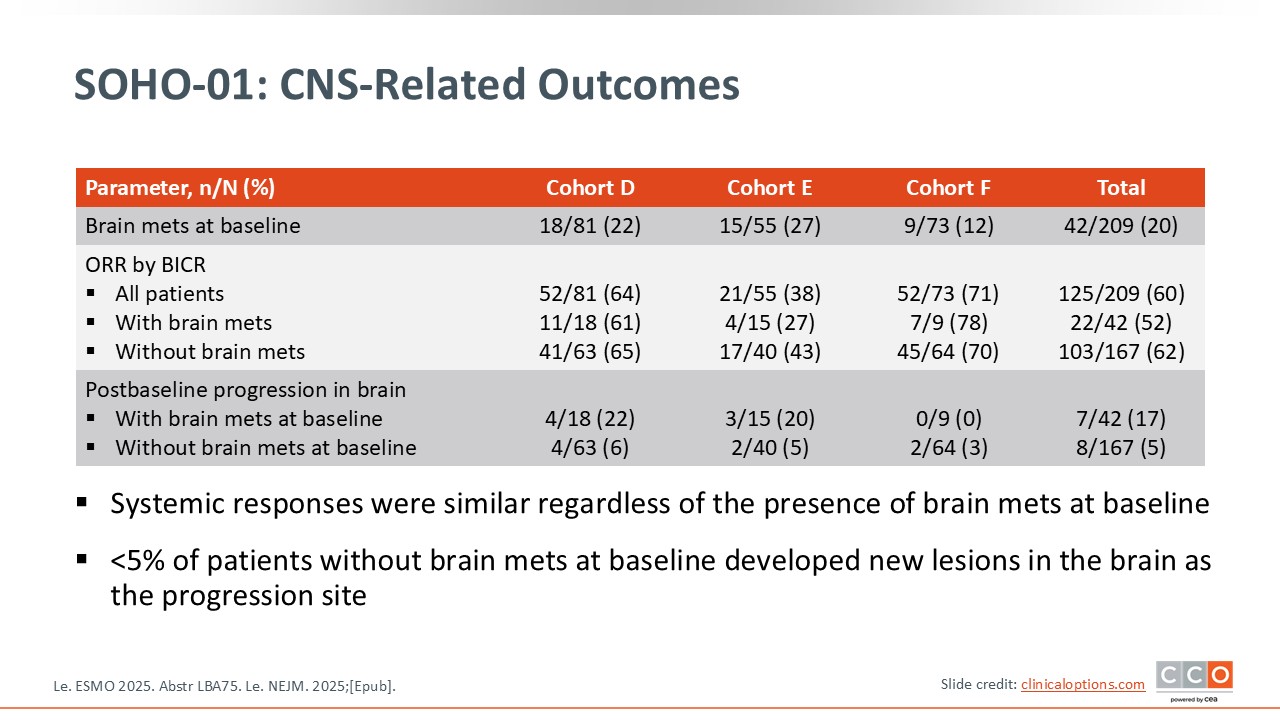
One important consideration is the inclusion of patients with brain metastases. We have limited information regarding whether these were untreated or previously treated brain metastases, and the study did not specifically evaluate intracranial responses. This remains a critical issue in this patient population, as brain metastases are common and clinically meaningful. For that reason, I would remain cautious when interpreting the results for patients with brain metastases, as this aspect was not clearly addressed in the trial.
Overall, SOHO-01 was designed to better define where sevabertinib might fit in the treatment landscape and to clarify its activity across different clinical scenarios in HER2-mutated disease, while keeping in mind these limitations.
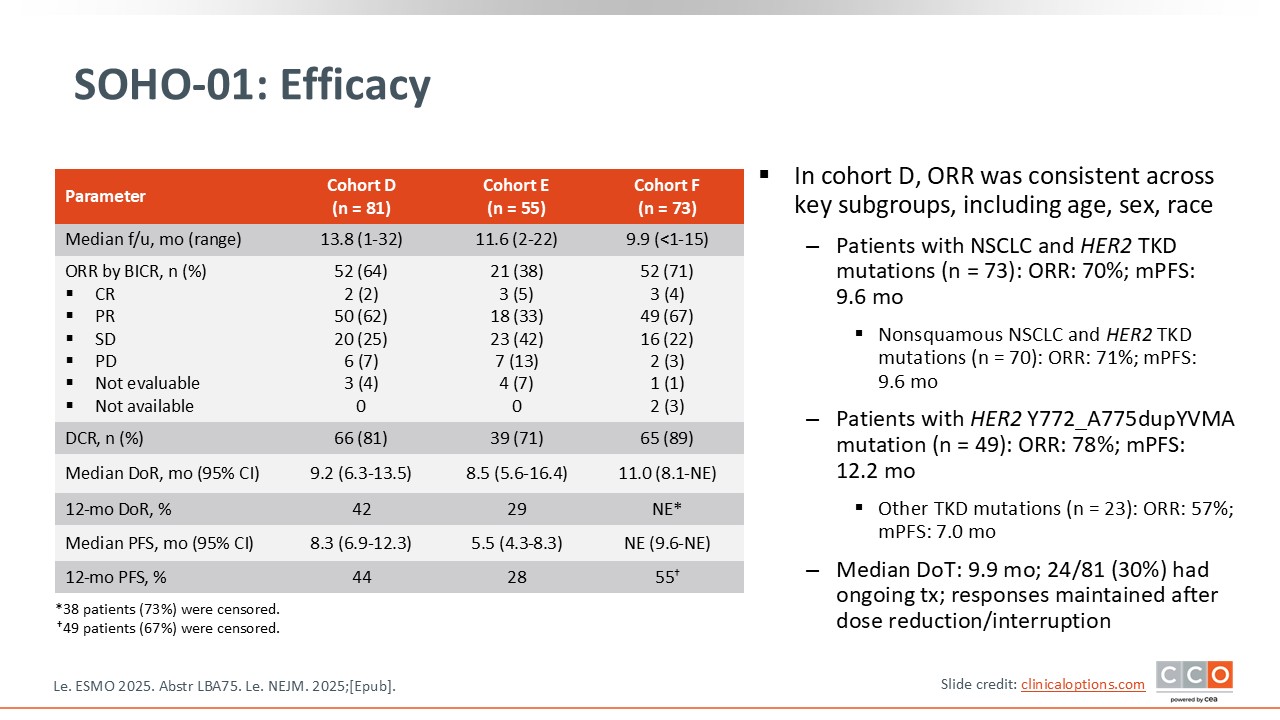
SOHO-01: Efficacy
David Planchard, MD, PhD:
The key finding was the impressive response rates observed across the 3 treatment cohorts. In particular, cohort D, which included previously treated patients who had not received any HER2-targeted agents, showed a remarkable response rate of 64%. This is quite notable for this population. The PFS was also encouraging, at 8.3 months, suggesting that the drug is highly active in this setting.
Even among patients who had previously been treated with a HER2-targeted ADC such as trastuzumab deruxtecan, the results remained promising. In this group, cohort E, the response rate was 38%, with a median PFS of 5.5 months. Demonstrating this level of activity after prior HER2-targeted therapy is particularly noteworthy.
The results from cohort F, the treatment-naive population, are perhaps the most striking. As shown, the response rate reached 71%, and the median duration of response was 11 months. These outcomes highlight the strong potential of this agent when used earlier in the treatment course.
SOHO-01: CNS-Related Outcomes
As previously mentioned, I would be cautious when interpreting the data for patients with brain metastases. The study reports ORRs in patients with and without brain metastases, but it does not provide specific intracranial response data. What we truly need to assess in this population are intracranial response rates and intracranial PFS, neither of which were presented for the various cohorts in this phase II trial at ESMO.
Given this limitation, we cannot draw any conclusions about intracranial efficacy at this time, and caution is warranted when considering the drug’s activity in patients with brain metastases.
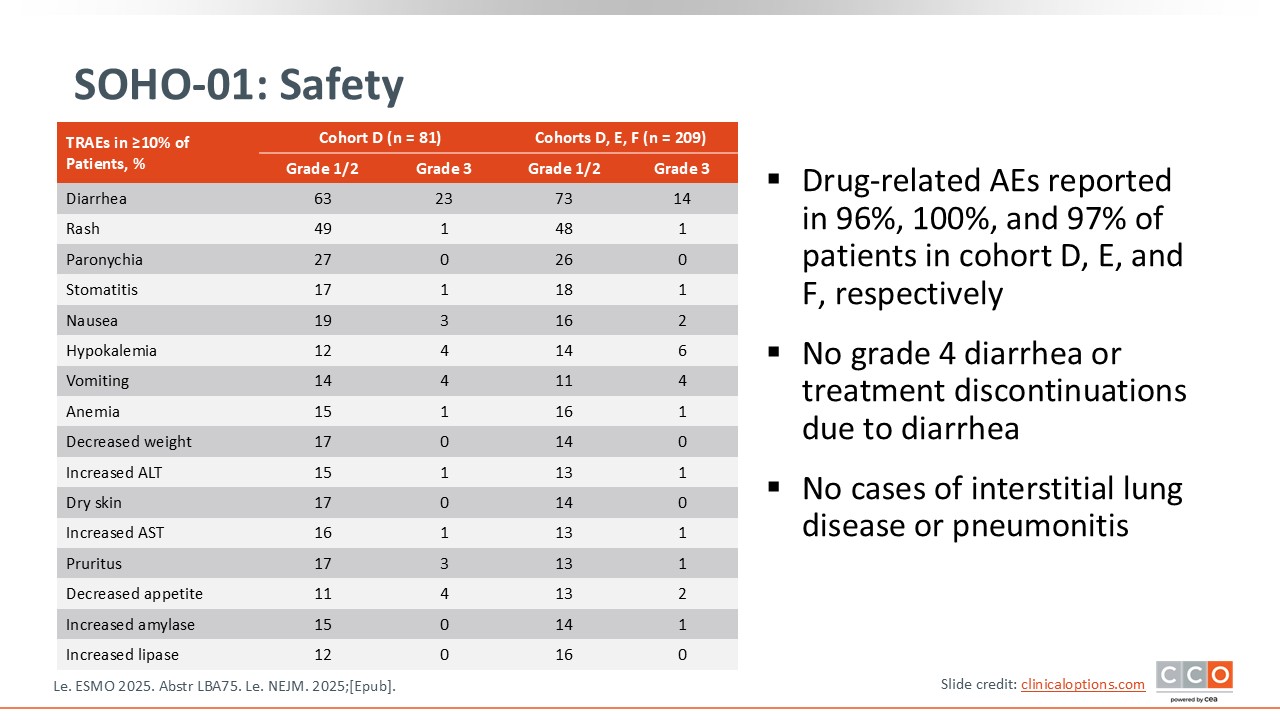
SOHO-01: Safety
Regarding safety, there are notable toxicities that need to be anticipated, particularly gastrointestinal effects. Diarrhea was observed in 86% of patients, mostly grade 1 or 2. Even low-grade diarrhea can significantly affect patients, especially when it occurs daily, and the study did not report the duration of these episodes. Given how frequent this toxicity is, it is essential to be proactive in managing and monitoring diarrhea with this compound.
On a positive note, no cases of ILD or pneumonitis were reported. This is reassuring, as pulmonary toxicity is a known concern with some targeted therapies. In summary, diarrhea requires careful anticipation and management, while the absence of ILD represents a favorable aspect of the safety profile.
Beamion LUNG-1: Phase I Study of Zongertinib as First-line Treatment for Advanced HER2-Mutant NSCLC
David Planchard, MD, PhD:
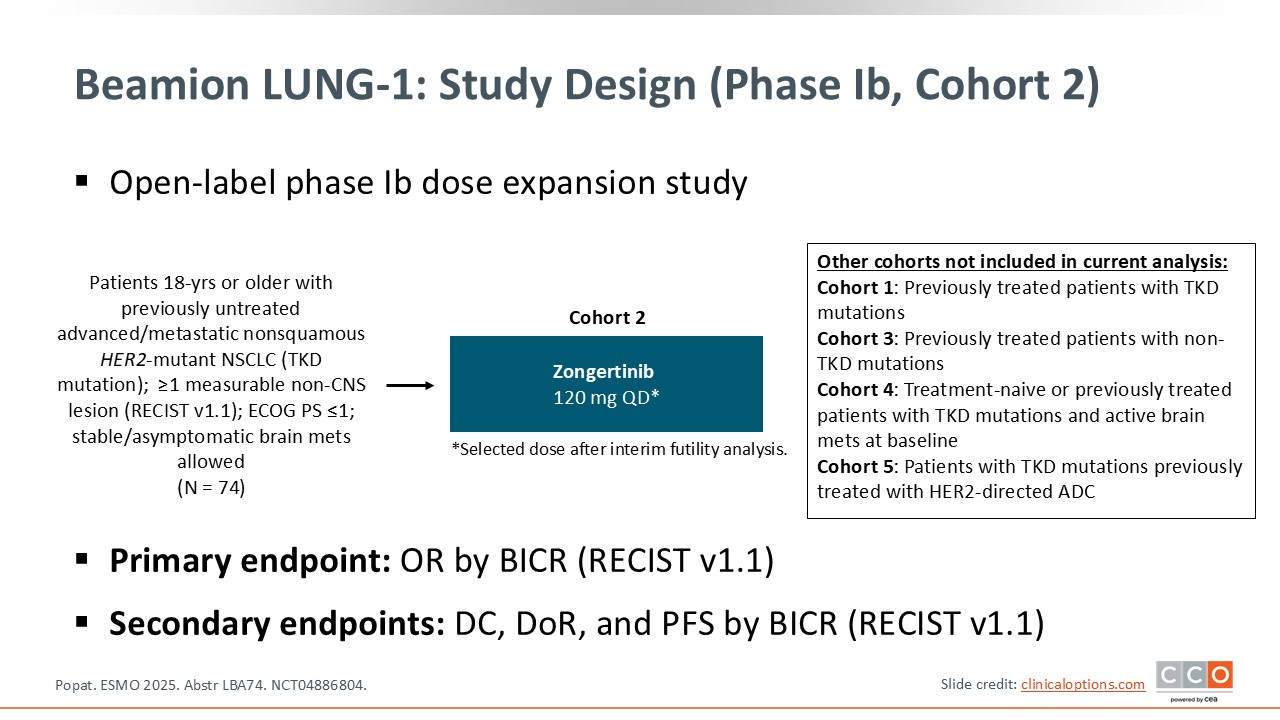
The key question of the Beamion LUNG-1 study was to evaluate whether zongertinib provides meaningful clinical benefit, specifically in terms of response rate, for patients with HER2-mutant NSCLC who are treatment naive and have not previously received HER2-targeted therapy.9 In other words, the trial was designed to assess how effective this drug could be as a first-line option for HER2-mutated NSCLC.
This was a nonrandomized phase II study that enrolled 74 patients. Individuals with stable, asymptomatic brain metastases were allowed to participate. However, caution is warranted when interpreting the findings for patients with brain metastases, as the study does not provide detailed information on intracranial efficacy, nor does it clarify whether these metastases were previously treated or untreated. Because brain involvement is common and clinically significant in this population, the lack of specific intracranial data remains an important limitation.
Beamion LUNG-1: Objective Response (Primary Endpoint)
David Planchard, MD, PhD:
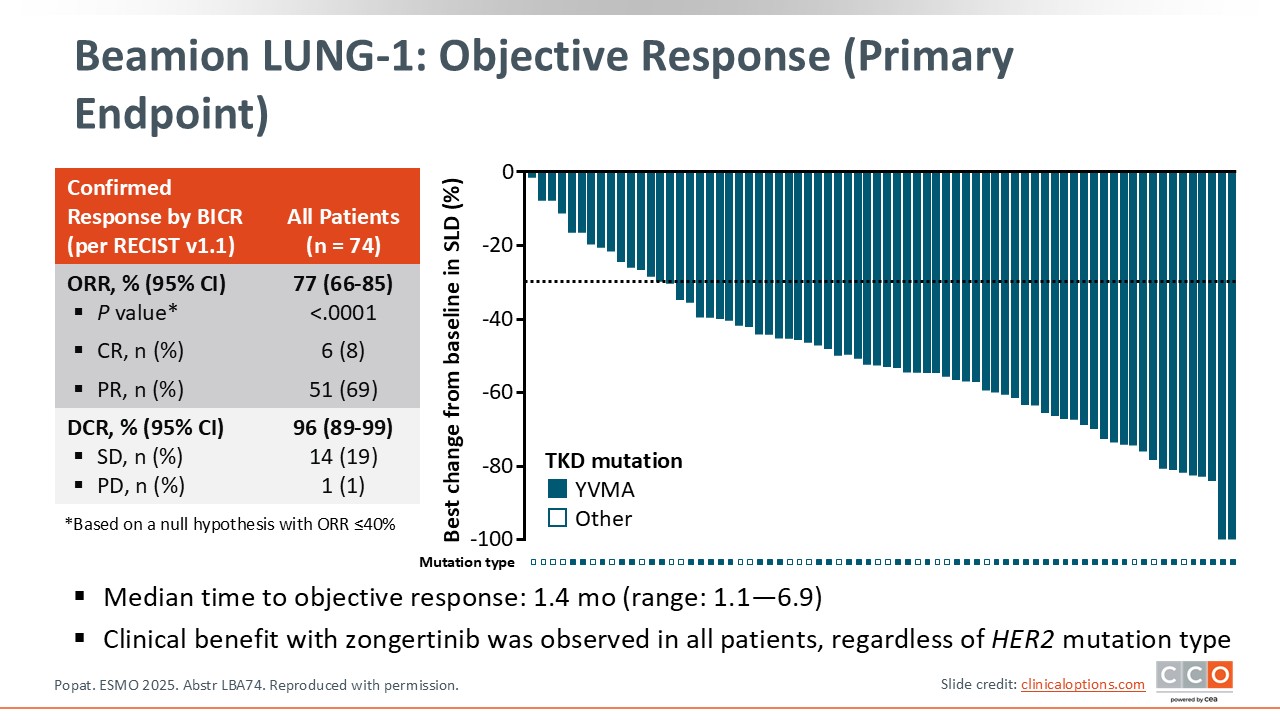
The primary endpoint was response rate, and the results were impressive—77%, supported by a striking waterfall plot. The activity of the drug appeared consistent across different types and locations of HER2 mutations, which is particularly encouraging.
The duration of response is still immature but appears potentially promising. As expected for this type of targeted therapy, responses occurred early, with a median time to objective response of 1.4 months. In most cases, patients responded by the time of the first CT scan, underscoring the impressive activity of the drug and suggesting the possibility of sustained benefit.
Beamion LUNG-1: DoR and PFS Rates
David Planchard, MD, PhD:
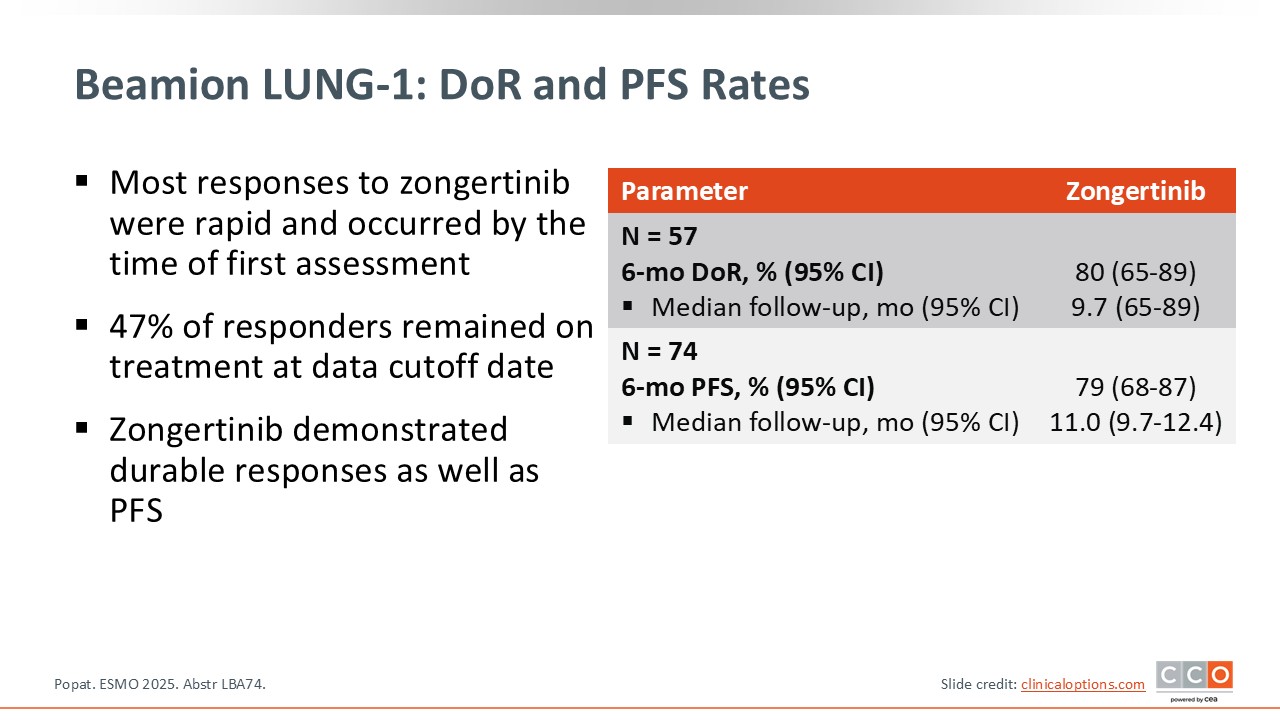
Data for both duration of response and PFS remain early. Even so, the results are encouraging: at 6 months, 79% of patients were progression free.
Beamion LUNG-1: Safety Summary
David Planchard, MD, PhD:
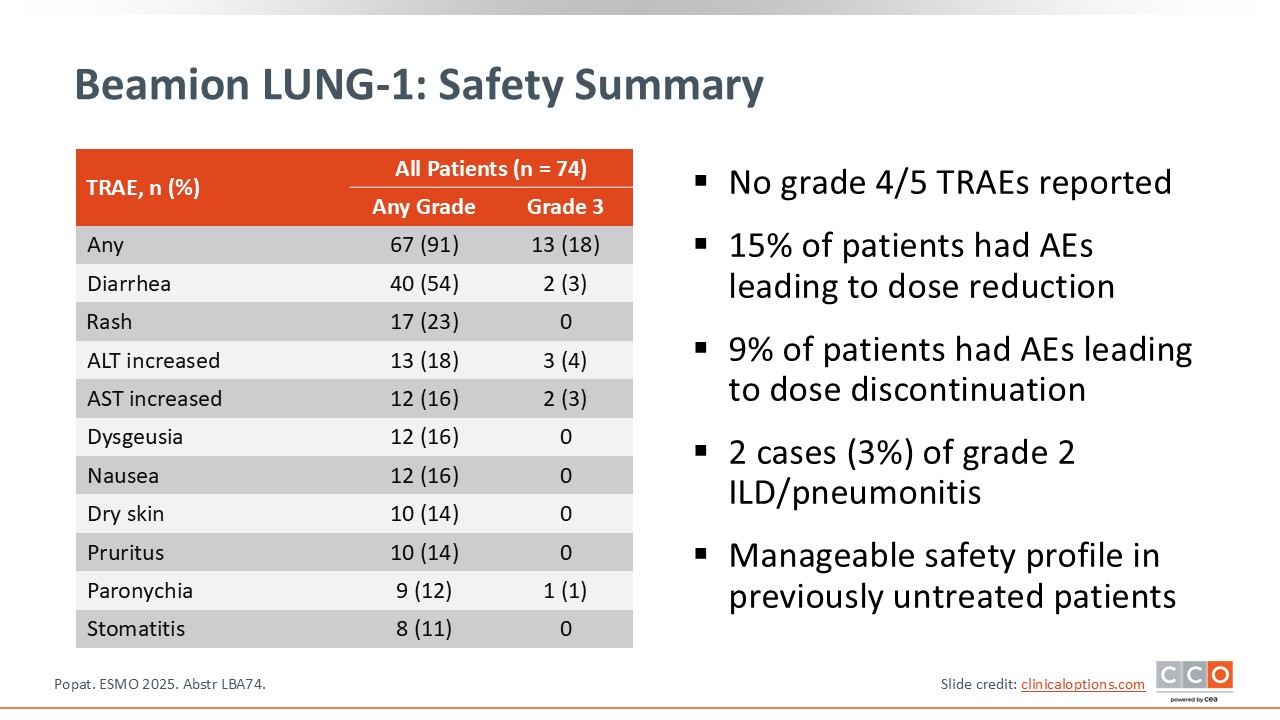
The safety profile appears favorable, especially when compared with some other agents in this space. Although gastrointestinal toxicity—primarily diarrhea—was observed, the incidence was mostly grade 1 or 2 and seemed lower than what has been reported with other agents in this disease setting. Early data suggest good overall tolerability with respect to both digestive and skin-related toxicities.
However, some caution is warranted. Two cases of ILD (approximately 3% of patients), both grade 2, were reported. Given the small sample size, it will be important to confirm in larger studies that pulmonary toxicity remains uncommon with this compound.
In summary, the drug shows generally good tolerability with manageable diarrhea and rash, but vigilance regarding ILD is essential despite its currently low reported rate.
SOHO-1 and Beamion LUNG-1: Clinical Implications
David Planchard, MD, PhD:
When looking at the data from SOHO-01 with sevabertinib and Beamion LUNG-1 with zongertinib, what strikes me first is how convincingly both studies show the activity of next-generation HER2 TKIs in HER2-mutated NSCLC, particularly when we are dealing with TKD mutations. In SOHO-01, for instance, we see a median PFS of approximately 8 months in previously treated patients and close to 12 months in the treatment-naive cohort, acknowledging, of course, that the first-line data still need time to mature.
Luis Paz-Ares, MD, PhD:
I completely agree. Both drugs are very active in HER2 TKD–mutated disease, and sevabertinib in SOHO-01 especially shows that strength in the untreated population. But the safety profile is not identical. Sevabertinib carries more EGFR-related toxicity—diarrhea, rash, the classic pattern you see with less selective inhibition. It is manageable, but certainly more pronounced than what we see with zongertinib.
David Planchard, MD, PhD:
Yes, and that’s exactly where Beamion LUNG-1 differentiates itself. Zongertinib appears to deliver similar efficacy, though we both know cross-trial comparisons are risky. What is more concrete is the tolerability profile. The cleaner, more HER2-selective inhibition with zongertinib likely explains the improved safety, and that could matter significantly as these agents advance into frontline practice.
Luis Paz-Ares, MD, PhD:
Absolutely. And both drugs are already being evaluated in first-line randomized trials, which will be critical. Sevabertinib’s SOHO-02 trial (NCT06452277) and zongertinib’s Beamion LUNG-2 (NCT06151574) are really going to determine the proper positioning. What we have now is compelling, but nonrandomized.
David Planchard, MD, PhD:
Beyond comparing the 2 TKIs, there is also the broader question of how they fit relative to HER2-directed ADCs like trastuzumab deruxtecan. Based on what we’re seeing, I tend to think that patients with HER2 TKD mutations are more likely to benefit from TKIs, whereas for non-TKD alterations, ADCs may remain equally strong options.
Luis Paz-Ares, MD, PhD:
That mirrors my impression as well. For true TKD-driven disease, the TKIs seem particularly effective. But we still need more clarity, especially regarding activity in brain metastases—which remains an unresolved issue—in particular for sevabertinib in SOHO-01.
David Planchard, MD, PhD:
Exactly. That is why even though both datasets are convincing and could reshape the treatment landscape, we still need solid randomized evidence to establish their definitive roles. For now, both sevabertinib and zongertinib stand as highly effective agents likely to move forward in frontline care, but their ultimate adoption will hinge on the outcomes of those ongoing phase III trials.
Luis Paz-Ares, MD, PhD:
The field is certainly moving quickly. Both sevabertinib and zongertinib were recently approved by the FDA for patients with locally advanced or metastatic NSCLC with HER2 TKD-mutated tumors identified with an approved test who have received previous systemic therapy.10,11 With these results, HER2-mutated NSCLC is finally gaining the targeted strategies it has long needed.