CME
Key Studies in Lung Cancer: Independent Conference Coverage of ESMO 2025
European Learners: 1.50 EBAC® CE Credit
Physicians: Maximum of 1.50 AMA PRA Category 1 Credits™
Released: December 16, 2025
Expiration: June 15, 2026
Activity
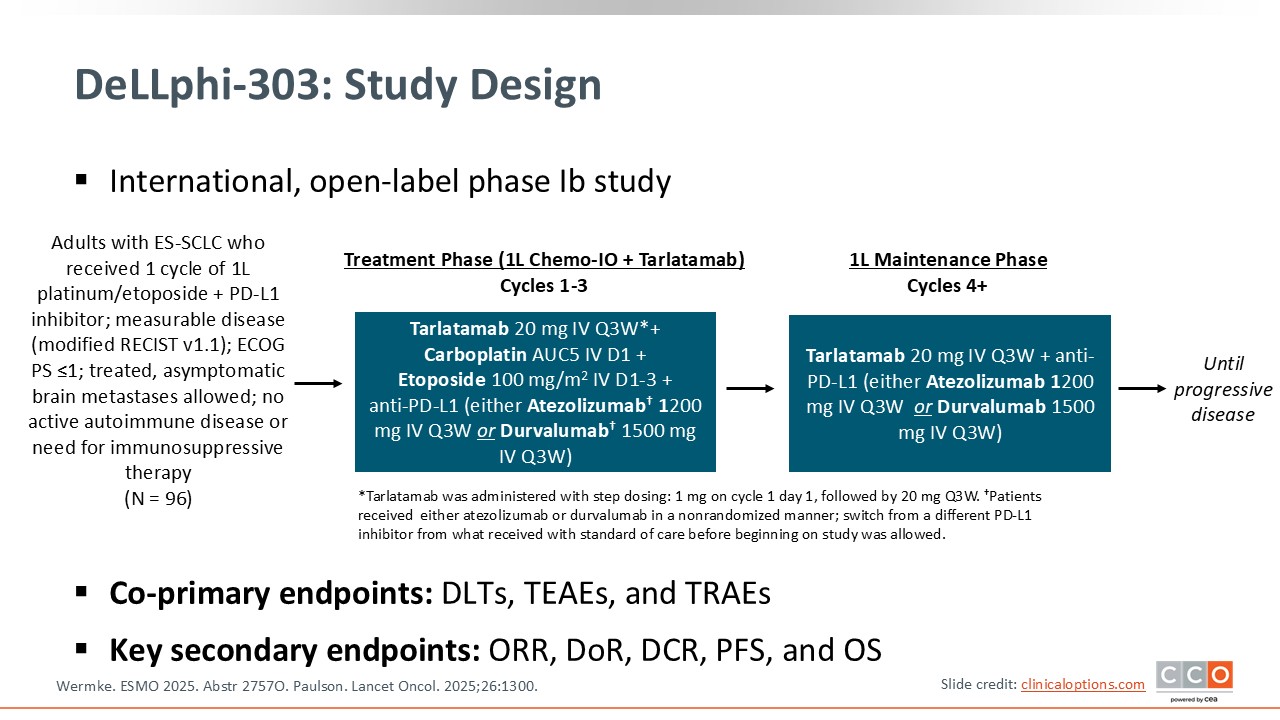
DeLLphi-303: Phase Ib Study of 1L SoC Chemoimmunotherapy + Tarlatamab in Extensive-Stage Small-Cell Lung Cancer
Luis Paz-Ares, MD, PhD:
The phase Ib DeLLphi-303 study is investigating the use of the T-cell engager tarlatamab as part of first-line therapy for patients with extensive-stage (ES)-small-cell lung cancer (SCLC).12 Tarlatamab is a DLL3-targeting T-cell engager and earlier clinical studies have already indicated that it can significantly alter the natural history of SCLC. In phase I and II trials, tarlatamab produced objective responses in approximately 35% to 40% of previously treated patients with ES-SCLC, with a surprisingly long median OS of nearly 18 months—a notable improvement in a disease known for its aggressive course.
More recently, a phase III trial demonstrated that tarlatamab used in the second-line setting improved survival compared with standard salvage chemotherapies such as topotecan, lurbinectedin, or amrubicin, with a hazard ratio of 0.6, further reinforcing its therapeutic potential.13
The DeLLphi-303 study presented at ESMO is the first to evaluate tarlatamab in the frontline setting for ES-SCLC, combining it with standard chemoimmunotherapy. Patients received 1 initial cycle of chemotherapy plus immunotherapy (atezolizumab or durvalumab). Beginning with cycle 2, tarlatamab was added to chemoimmunotherapy for 3 induction cycles. After completing the 4-cycle induction phase, patients continued on maintenance immunotherapy plus tarlatamab.
This trial is critical because it explores whether incorporating tarlatamab earlier in the treatment course rather than after relapse can further improve outcomes in newly diagnosed ES-SCLC, a setting where advances have been extremely limited.
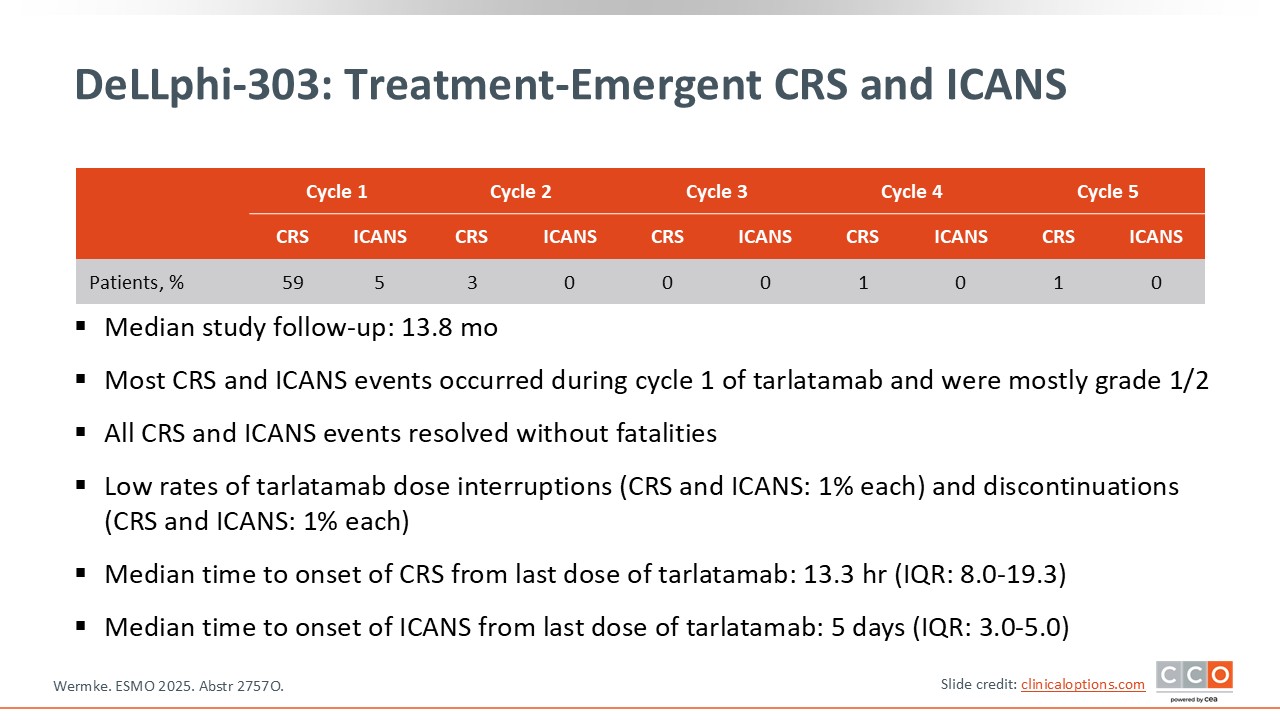
DeLLphi-303: Treatment-Emergent CRS and ICANS
Luis Paz-Ares, MD, PhD:
The key point reported from this trial is that no new safety signals were observed. Cytokine release syndrome (CRS) occurred in approximately 59% of patients, predominantly grade 1-2 events. More than 30% of patients experienced grade 1 CRS, roughly 20% had grade 2, and only approximately 1% had grade 3 CRS.
Immune effector cell–associated neurotoxicity syndrome (ICANS) was reported in fewer than 5% of patients and was almost entirely limited to grade 1-2 severity.
Of importance, these toxicities occurred mainly during the initial treatment cycles, with a few cases extending into cycle 2, but they were rare beyond that point. Overall, the safety profile showed no unexpected findings, which is reassuring.
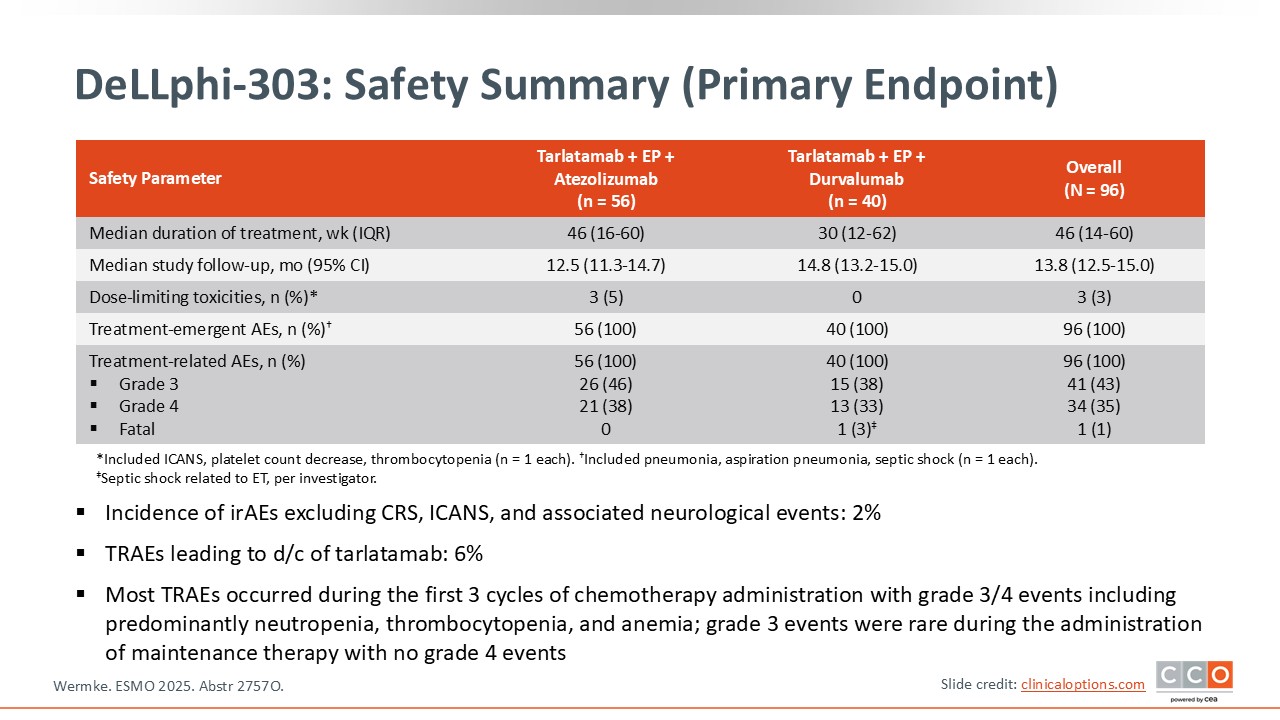
DeLLphi-303: Safety Summary (Primary Endpoint)
Luis Paz-Ares, MD, PhD:
In addition to CRS and ICANS, we also observed the expected toxicities associated with chemotherapy, as well as some dysgeusia and asthenia, which are characteristic side effects of tarlatumab.
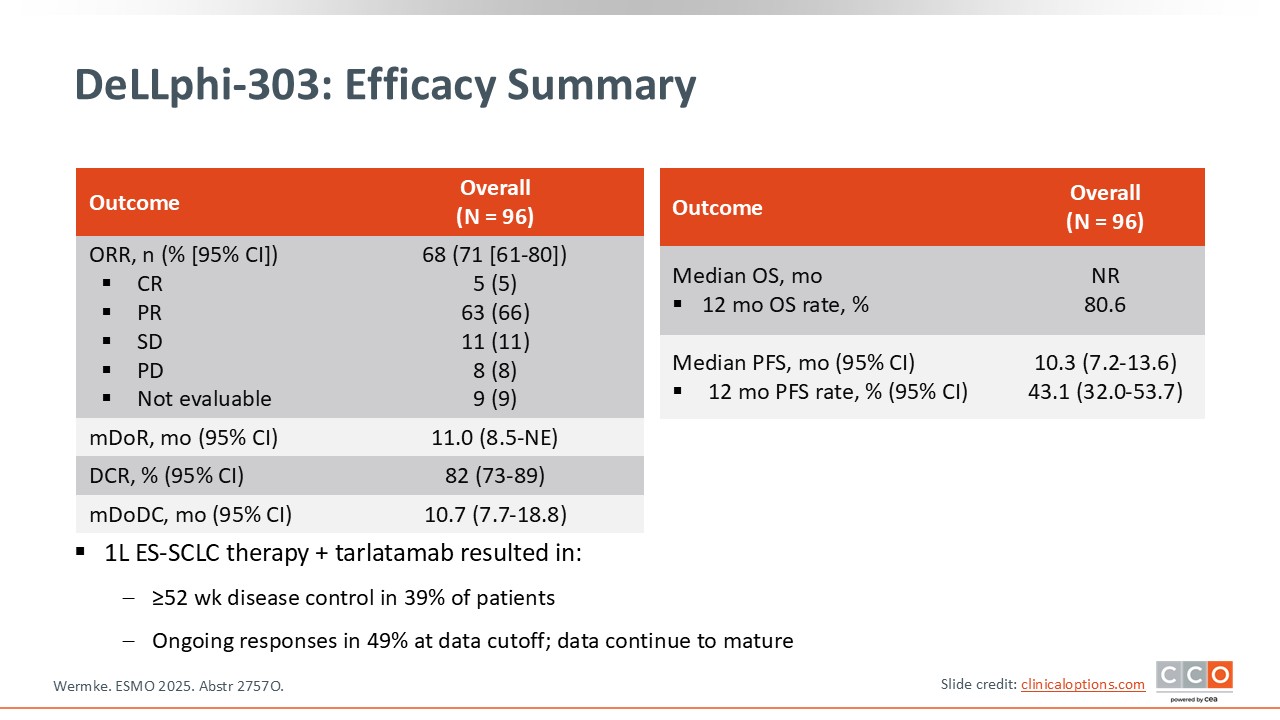
DeLLphi-303: Efficacy Summary
Luis Paz-Ares, MD, PhD:
Regarding efficacy, the data were quite compelling. The ORR was similar to what we typically expect from chemotherapy plus immunotherapy, so there was no major improvement in that specific metric. However, there was a clear improvement in median PFS compared with historical expectations.
At 1 year after initiating treatment, 43% of patients remained progression free, and of importance, OS at 1 year reached 81%. These are remarkably encouraging results.
DeLLphi-303: Clinical Implications
David Planchard, MD, PhD:
The DeLLphi-303 study really represents a potentially major shift in the management of ES-SCLC. We already know that tarlatamab has shown clear, practice-changing benefit in the second-line setting, significantly improving both PFS and OS compared with chemotherapy and establishing itself as the new standard of care after relapse.13
What DeLLphi-303 does is extend that investigation into the first-line setting. Here, tarlatamab was combined with standard chemotherapy and immunotherapy. Although the data remain immature, the early signals are highly compelling: the 1-year OS rate was roughly 80%, and approximately 43% of patients were progression free at 1 year. These numbers appear to exceed what we typically achieve with chemoimmunotherapy alone. Given how aggressive SCLC is, seeing this scale of benefit is really quite striking.
As expected with T-cell engagers, CRS and ICANS were the main toxicities. But of importance, these events were concentrated in the first cycle of tarlatamab and became far less frequent thereafter, which is consistent with what we’ve observed across this entire drug class. Once patients got through those initial infusions, the regimen was very manageable.
Because ES-SCLC has such a poor prognosis, any treatment that can meaningfully prolong survival is notable. DeLLphi-303 is admittedly a small, nonrandomized study, but the results strongly suggest that tarlatamab could become not just our second-line standard, which it already is, but potentially a future first-line standard in combination with chemoimmunotherapy as well. The randomized trials now underway will be essential, but expectations across the field are very high that we will see a similar benefit when used upfront.
Luis Paz-Ares, MD, PhD:
I fully agree; these data are very encouraging, and they are consistent with other recent findings. At the 2025 World Conference on Lung Cancer, we saw results where tarlatamab was added during the maintenance phase after 4 cycles of chemoimmunotherapy. That study showed a 1-year survival rate of 82%, with approximately 34% of patients progression free at 1 year, which again compares very favorably with historical expectations.14
We also saw the DAREON-8 study at ESMO, which evaluated obrixtamig, another DLL3-targeted T-cell engager, in combination with chemotherapy and atezolizumab from cycle 1.15 Patients then continued on obrixtamig plus atezolizumab during maintenance. The safety profile was very consistent with what we have seen with tarlatamab: no new CRS, ICANS, or other unexpected toxicities. The response rate was 68%, and the 9-month PFS rate was approximately 52%. These results closely mirror the outcomes from tarlatamab plus chemoimmunotherapy in DeLLphi-303, reinforcing that DLL3-directed T-cell engagement may represent a class effect with real first-line potential.
Overall, having 2 different DLL3-targeting agents producing such promising early-phase data is remarkable. The phase III trials will be critical, but there is real enthusiasm that T-cell engagers could redefine the treatment landscape for ES-SCLC.
In my experience, the safety profile has been extremely consistent across third-line, second-line, first-line, and first-line maintenance settings. We see CRS in approximately 50% to 60% of patients, roughly 30% grade 1 and 20% grade 2 with grade 3 events being rare. ICANS occurs in fewer than 10% of patients and is almost always low grade. Most events are confined to the first cycle, with occasional isolated cases in cycle 2.
For managing these toxicities, the key is education—not just for physicians and nurses, but for patients, families, and caregivers. Historically we hospitalized patients for their first 2 or 3 infusions. Today, if patients live close by and have 24-hour caregiver support, we often observe them for about 8 hours post infusion and then allow them to go home.
We use prophylactic dexamethasone routinely. For grade 1 events, additional steroids or antipyretics may be used depending on whether the patient is at home or in the hospital. For grade 2 CRS, we provide dexamethasone and IV fluids, and if hypotension doesn’t respond to fluids or if oxygen is needed, we escalate accordingly. For grade 2 CRS not improving with volume expansion, we initiate tocilizumab.
Before starting therapy, our ICU colleagues are always alerted. If a patient exhibits persistent grade 2 toxicity, we transition them to ICU monitoring to ensure rapid intervention.
Conclusions
Luis Paz-Ares, MD, PhD:
Overall, I would say there were several very interesting datasets this year. Perhaps not as practice changing across the board as in some previous meetings, but still quite meaningful. For me, the standout studies were HARMONi-6 with ivonescimab in squamous NSCLC, the OptiTROP trial evaluating sacituzumab tirumotecan in EGFR-mutant disease progressing after third-generation TKIs, and, of course, the first-line data with tarlatamab in combination with chemoimmunotherapy. Each of these represents real progress and helps signal where the field may be heading.
David Planchard, MD, PhD:
I agree completely. In fact, I thought ESMO 2025 was an exceptionally exciting meeting for lung cancer. We saw 2 randomized phase III trials—ivonescimab and sacituzumab tirumotecan—both delivering positive PFS results and potential OS benefit. It is truly remarkable to see 2 potentially practice-changing trials presented back to back, each targeting major patient populations: squamous NSCLC and EGFR-mutated disease.
Beyond the randomized data, we also saw compelling early-phase results with zongertinib and sevabertinib in HER2-mutated NSCLC. This is a population where we currently lack approved targeted therapies or HER2-directed TKIs in Europe though both agents were recently approved in the US, and the activity shown by both agents was quite striking. It really suggests that we may finally be moving toward effective targeted options for these patients as well.
Luis Paz-Ares, MD, PhD:
Yes, absolutely. When you look across EGFR-mutated disease, squamous histology, and HER2-mutated tumors, the breadth and quality of positive data presented this year were truly impressive. Even if not every dataset is immediately practice changing, the overall momentum is clearly building. We are seeing meaningful advances across multiple molecular subsets.
David Planchard, MD:
Exactly. The field feels energized. With randomized phase III successes, highly promising targeted agents emerging, and continued innovation in immunotherapy and ADCs, this year really reflects a moment of genuine progress for the lung cancer community.