CME
Key Studies in Lung Cancer: Independent Conference Coverage of ESMO 2025
European Learners: 1.50 EBAC® CE Credit
Physicians: Maximum of 1.50 AMA PRA Category 1 Credits™
Released: December 16, 2025
Expiration: June 15, 2026
Activity
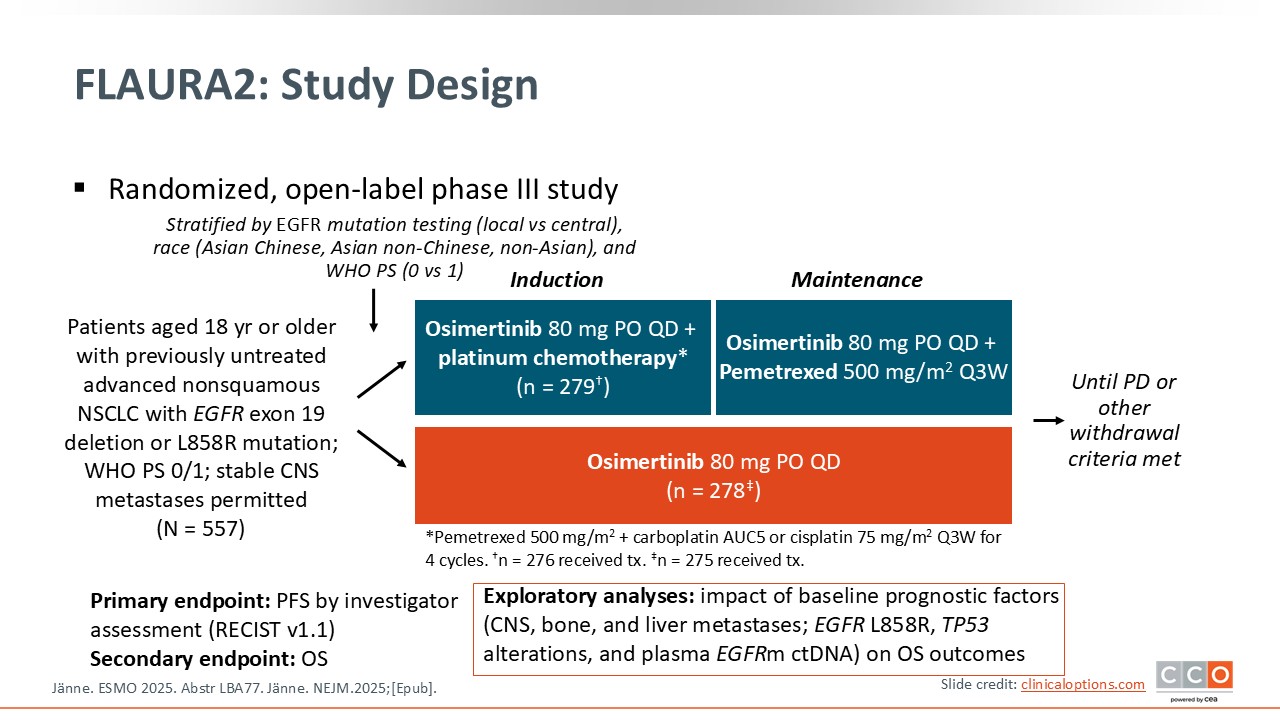
FLAURA2: OS Outcomes by Baseline Prognostic Factors in EGFR-Mutated NSCLC Receiving 1L Osimertinib ± Platinum-Based CT
Luis Paz-Ares, MD, PhD:
The key question being asked in the FLAURA2 trial is whether combining chemotherapy with osimertinib provides a meaningful survival advantage over osimertinib alone for patients with EGFR-mutated advanced NSCLC—and, critically, in this report whether certain clinical or molecular subgroups derive more or less benefit from this intensified approach.4
This question is important because the mature OS results from FLAURA2 have now shown that the chemotherapy plus osimertinib reduces the risk of death by approximately 25% compared with osimertinib alone (HR: 0.77). Given such a significant improvement, HCPs naturally want to understand which patients truly need the addition of chemotherapy, a treatment escalation that carries additional toxicity and treatment burden.
This exploratory subanalysis begins to address this by examining whether the survival benefit varies across clinically relevant subgroups including patients with or without baseline CNS, bone, or liver metastases; those with different EGFR mutation types (exon 18 vs exon 21); those with or without TP53 comutations; and those with or without detectable EGFR ctDNA at baseline.
FLAURA2 Exploratory OS Analyses: Prognostic Subgroups
Luis Paz-Ares, MD, PhD:
This analysis showed that the benefit of chemotherapy plus osimertinib was broadly consistent across all evaluated subgroups. No patient population appeared to gain disproportionately more or less benefit, suggesting that the survival advantage of adding chemotherapy applies widely across the typical spectrum of patients with EGFR-mutated NSCLC. Although there was a hint that patients with liver metastases might experience slightly greater benefit than those without, the confidence intervals were largely overlapping, indicating no meaningful difference. Altogether, these results support the robustness and generalizability of the FLAURA2 findings and reinforce that patients appear to benefit regardless of baseline prognostic factors.
FLAURA2: Clinical Implications
David Planchard, MD, PhD:
With the mature FLAURA2 data, I think it is fair to say the standard of care in first-line EGFR-mutated NSCLC has truly shifted. The combination of osimertinib plus platinum-based chemotherapy not only improved PFS but also OS, establishing it as the preferred regimen moving forward.
Luis Paz-Ares, MD, PhD:
I completely agree. The evidence shows that combination therapy simply provides better survival expectations than osimertinib alone, whether we’re talking about the FLAURA2 regimen or even MARIPOSA with lazertinib plus amivantamab.5 What is striking is that the subgroup analyses do not identify any population that fails to benefit. These traditional prognostic indicators just aren’t predictive here; each patient population saw benefit.
David Planchard, MD, PhD:
Yes. Even patients with the highest-risk disease biology—exon 21 mutations, brain metastases, liver metastases, or detectable plasma EGFR mutations—showed substantial benefit. In fact, those with the poorest prognostic features may gain the most, but the key point is that the survival advantage was consistent across all subgroups. So we shouldn’t restrict this approach only to high-risk patients.
Luis Paz-Ares, MD, PhD:
That is exactly how I approach it in my practice. My default is to recommend combination therapy for nearly all patients, because it offers the best survival outcomes. Of course, there are exceptions—patients with significant cardiac disease, renal dysfunction, or those who simply cannot tolerate chemotherapy. There are also patients who understandably prefer to avoid the added toxicity of chemotherapy or the need to come in every 3 weeks for infusions. For them, first-line osimertinib alone remains reasonable, but I’m clear that they may be losing an opportunity for improved outcomes.
David Planchard, MD, PhD:
Fortunately, the regimen is quite manageable. The toxicities of platinum chemotherapy are well known—mostly gastrointestinal and hematologic—and in FLAURA2, patients received only 4 cycles before transitioning to maintenance therapy with pemetrexed and osimertinib. The most challenging period is really the first 3 months, after which treatment becomes easier. As the trial allowed, HCPs can always de-escalate chemotherapy while continuing osimertinib if toxicity becomes an issue.
Luis Paz-Ares, MD, PhD:
Exactly. With flexible de-escalation and manageable toxicities, it is a feasible regimen for most patients. Given that the benefit is so broadly applicable, combination therapy should be strongly considered as the standard first-line option for eligible patients with EGFR-mutant NSCLC.
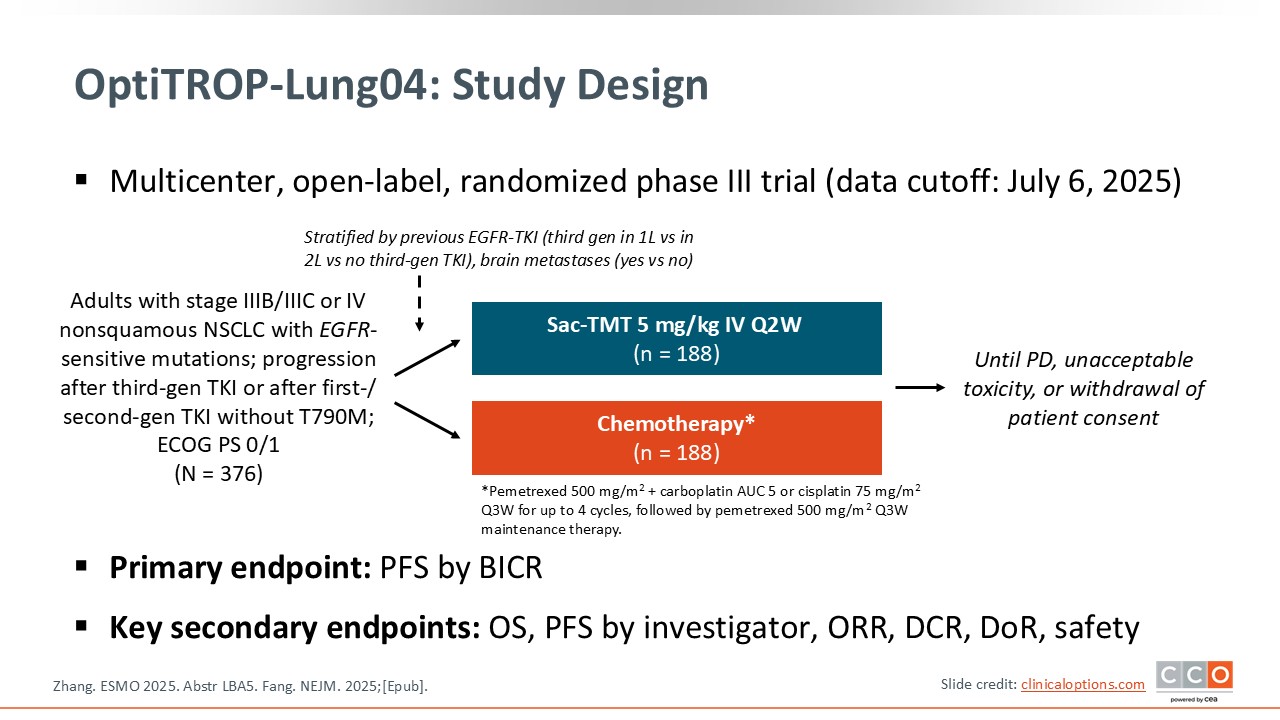
OptiTROP-Lung04: Phase III Trial of Sacituzumab Tirumotecan vs Chemotherapy in EGFRm NSCLC After Progression on EGFR-TKIs
David Planchard, MD, PhD:
The central question of the OptiTROP-Lung04 trial was whether sacituzumab tirumotecan, an antibody–drug conjugate (ADC) targeting TROP2, could improve clinical outcomes compared with standard platinum-based chemotherapy in patients with EGFR-mutated NSCLC who had progressed after treatment with a third-generation EGFR TKI.6
This question is particularly important because patients with EGFR-mutated NSCLC who experience disease progression following targeted therapy currently have limited effective treatment options. The standard of care in the second-line setting remains platinum-based chemotherapy, which provides only modest benefit. The development of ADCs represents an emerging therapeutic strategy that could potentially overcome resistance mechanisms and offer a novel, targeted approach for this patient population.
By evaluating sacituzumab tirumotecan in comparison with platinum-based chemotherapy, this trial aims to determine whether TROP2-directed ADC therapy can deliver superior PFS and OS outcomes, thereby establishing a new standard of care in the post–EGFR TKI setting for these patients.
Some aspects of this trial also warrant attention. First, the study was conducted exclusively in China, enrolling a Chinese patient population. This raises important considerations regarding the generalizability of the findings to non-Asian populations, as potential pharmacogenomic or biological differences may influence treatment response and tolerability.
Second, additional information is needed concerning the management of patients with brain metastases. Given that CNS involvement is common among individuals with EGFR-mutated NSCLC, it will be important to clarify the eligibility criteria for patients with active or progressive brain metastases, as well as the efficacy of sacituzumab tirumotecan in this patient subgroup. Future studies should address these aspects to better define the treatment’s potential across the full clinical spectrum of EGFR-mutated disease.
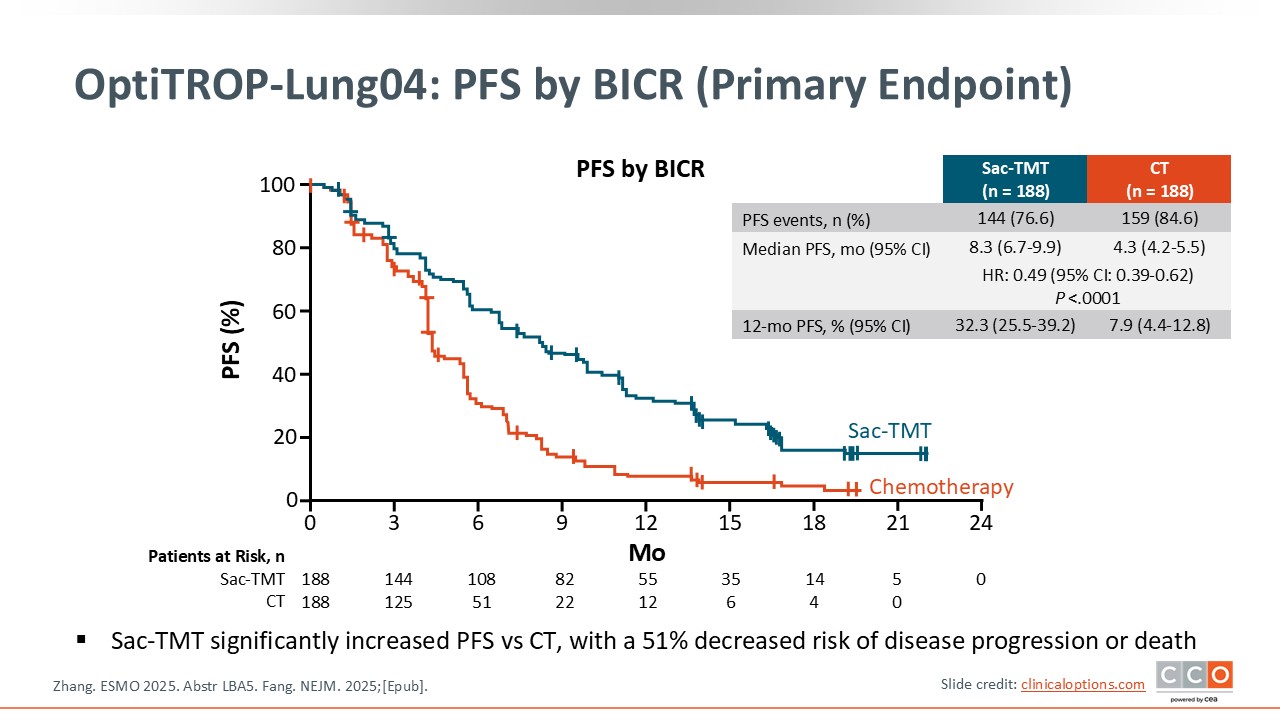
OptiTROP-Lung04: PFS by BICR (Primary Endpoint)
David Planchard, MD, PhD:
The key finding of the trial is that it met its primary endpoint, demonstrating a statistically and clinically significant improvement in PFS with sacituzumab tirumotecan vs platinum-based chemotherapy in patients with EGFR-mutated NSCLC who had progressed after treatment with a third-generation EGFR TKI.
The reported hazard ratio for PFS was 0.49, indicating a 51% reduction in the risk of disease progression or death with sacituzumab tirumotecan relative to chemotherapy. The Kaplan–Meier curves showed early and durable separation in favor of sacituzumab tirumotecan, suggesting a consistent benefit over time, although follow-up remains relatively short.
Overall, these results may establish sacituzumab tirumotecan as a superior second-line therapeutic option compared with standard chemotherapy in this population, representing an important step forward in the management of EGFR-mutated NSCLC after EGFR TKI failure.
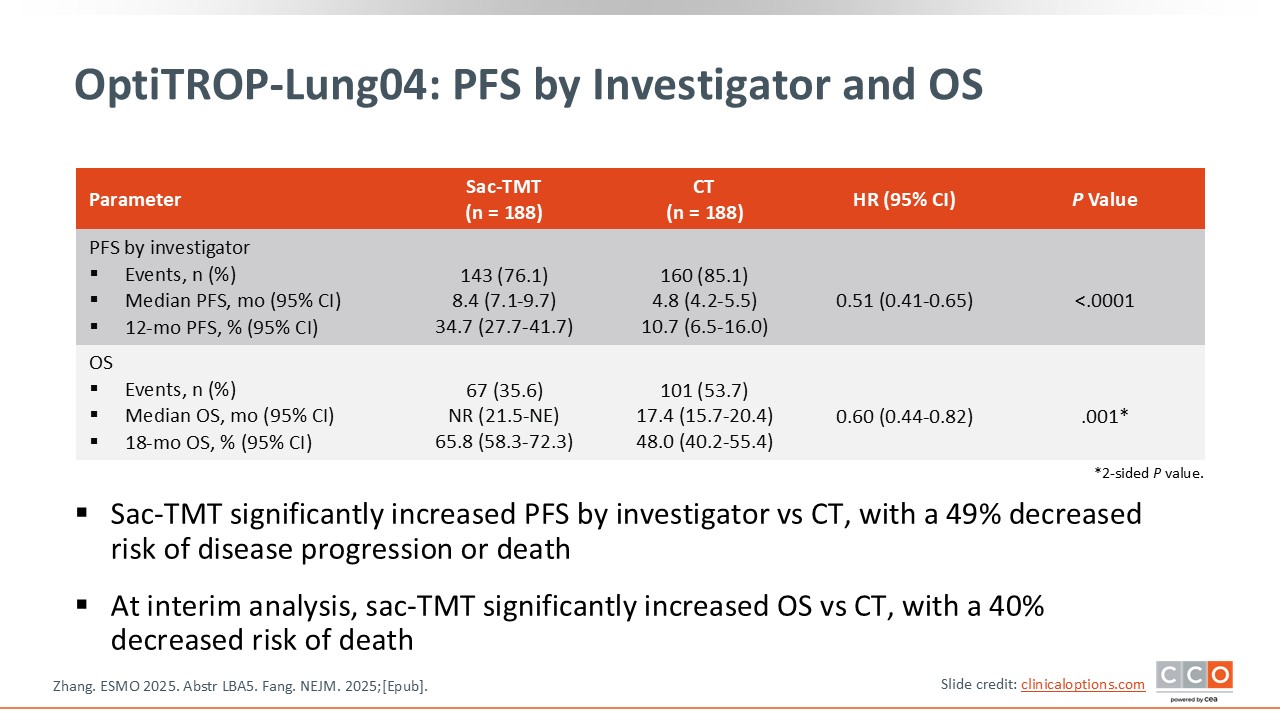
OptiTROP-Lung04: PFS by Investigator and OS
David Planchard, MD, PhD:
The second objective, likely one of the most important, is whether the trial shows that sacituzumab tirumotecan significantly improves OS. There is a favorable trend suggesting improved OS with sacituzumab tirumotecan, but the data are still immature and not yet statistically significant. Further follow-up is needed. However, the early results are encouraging, and it is reasonable to expect that the benefit seen in PFS may ultimately translate into an OS benefit. If confirmed, this could potentially change the standard of care for this patient population.
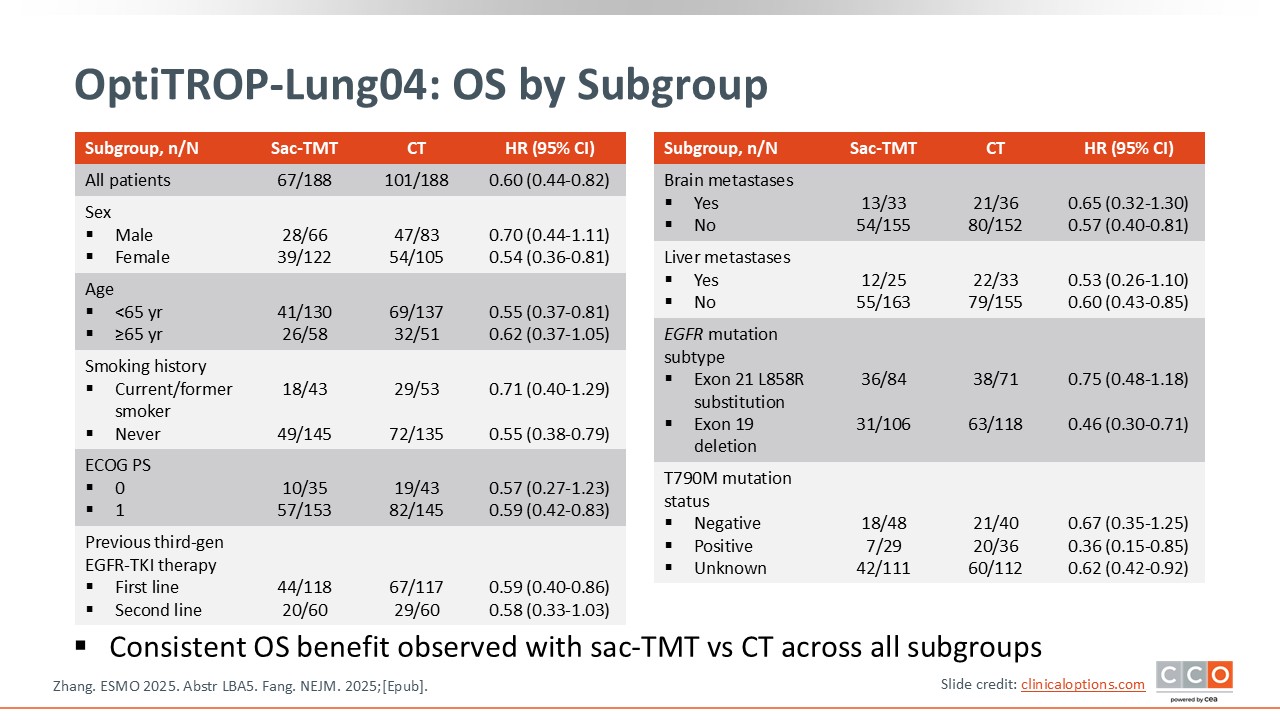
OptiTROP-Lung04: OS by Subgroup
David Planchard, MD, PhD:
Based on the available data, the subgroup analysis shows a PFS benefit across all evaluated subpopulations. This includes patients with different types of EGFR mutations, such as exon 19 deletions and exon 21 alterations, as well as patients with or without brain metastases. Overall, the results are highly encouraging, demonstrating a consistent benefit across EGFR-mutant subgroups.
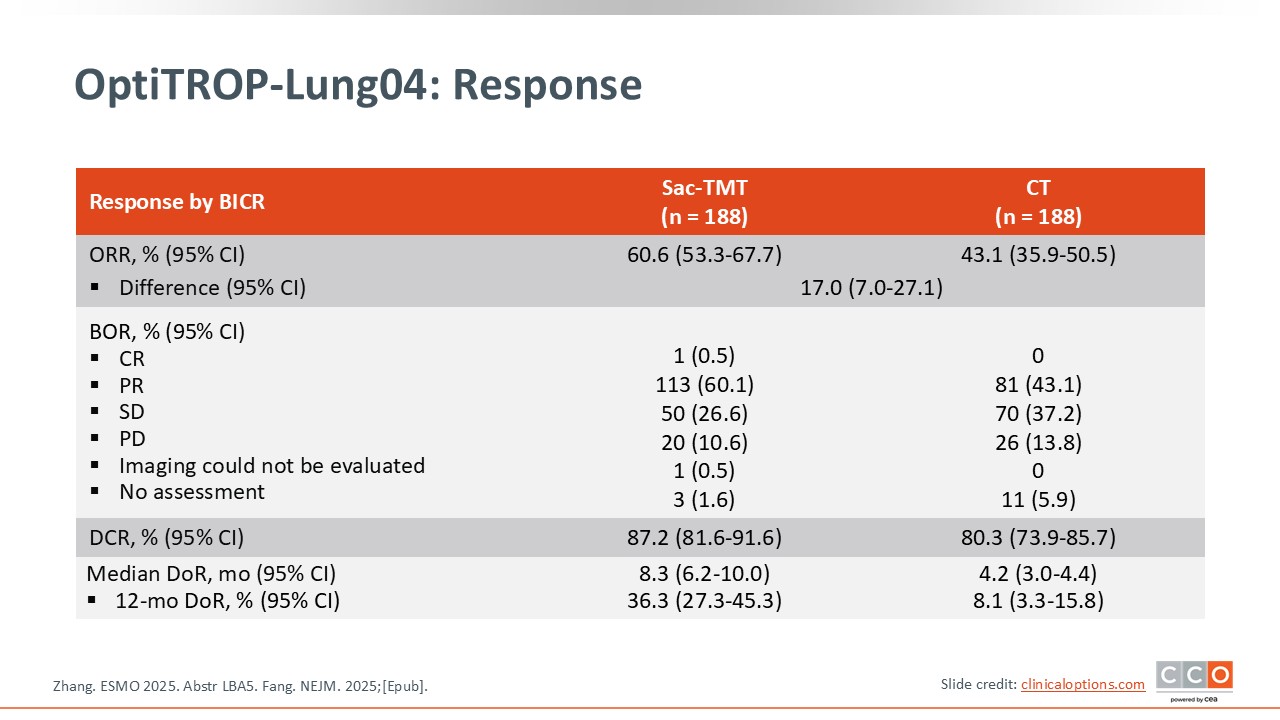
OptiTROP-Lung04: Response
David Planchard, MD, PhD:
In addition to the improvement in PFS, the trial also demonstrated a significant increase in response rate—60.6% vs 43.1%—which may offer meaningful clinical benefit for patients.
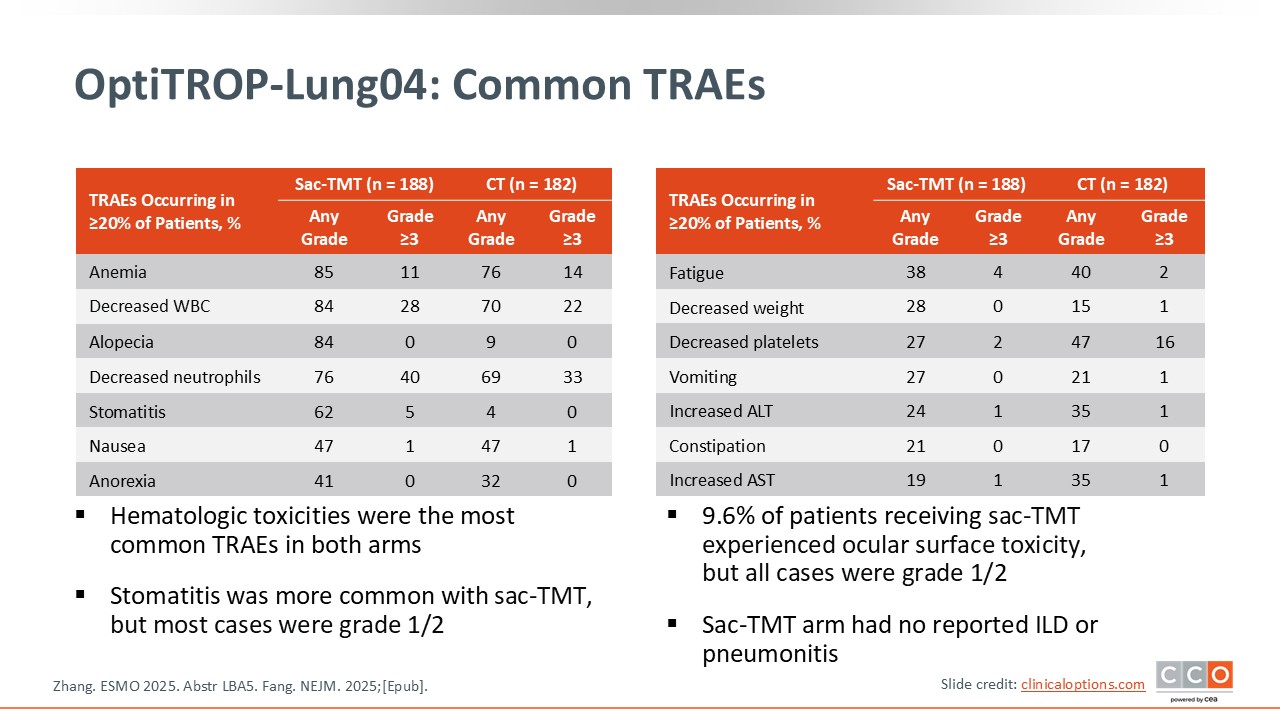
OptiTROP-Lung04: Common TRAEs
David Planchard, MD, PhD:
Regarding safety, the toxicity profile is generally similar to what we might expect with chemotherapy. Although this is an ADC, hematologic and gastrointestinal toxicities were observed, mostly grade 1 or 2. Of importance, this is not a non-toxic drug; its side effects are comparable to those seen with traditional chemotherapy.
The most notable safety concern is the high incidence of stomatitis. Stomatitis occurred in 62% of patients, mainly grade 1 or 2, but it can significantly affect patients’ quality of life. This toxicity appears to be characteristic of TROP2-targeting ADCs and has been observed with other agents in the same class. It is therefore essential to anticipate and manage stomatitis proactively to minimize its impact on patients.
In addition, ocular surface toxicity was reported in nearly 10% of patients, representing another class-specific consideration when using this type of ADC.
No cases of interstitial lung disease (ILD) or pneumonitis were observed in the trial.
OptiTROP-Lung04: Clinical Implications
Luis Paz-Ares, MD, PhD:
This is another very important trial. Although conducted exclusively in a Chinese population, the data clearly indicate that in patients who have progressed after a third-generation EGFR TKI, sacituzumab tirumotecan provides superior efficacy compared with standard platinum-based chemotherapy. We saw a significant improvement in PFS, with a hazard ratio of 0.49, and a meaningful interim OS benefit, with a hazard ratio of 0.6. The benefit was consistent across all the patient subsets analyzed.
From a safety perspective, the differences were modest—slightly more stomatitis and alopecia with the ADC, but nothing particularly concerning. Overall, the regimen was well tolerated.
Given the magnitude of benefit, I would be surprised if these results are not validated globally. Regulatory decisions may depend on confirmatory data, but the early signals are strong. Global phase III studies are already ongoing and recruiting patients, so broader evaluation is underway (NCT06305754 and NCT06074588).
David Planchard, MD, PhD:
I completely agree. These results are highly promising, but they are not yet practice changing. This drug does have the potential to become a new standard of care in the true second-line setting for patients with EGFR-mutated NSCLC who have already progressed on a third-generation EGFR inhibitor. However, to reach that point, we will need confirmation of an OS benefit, and of importance, validation in non-Chinese populations.
What stands out here is that this is the first randomized trial in the second-line setting showing that a TROP2-directed ADC can outperform platinum chemotherapy, which is a very challenging comparator. That’s quite different from how other ADCs in this space have been evaluated.
When we compare this therapy with datopotamab deruxtecan, we need to remember that although both agents target TROP2, they are distinct molecules with different payloads and properties. Datopotamab deruxtecan received US approval based on phase II, nonrandomized data and is positioned essentially as a third-line option after both an EGFR TKI and platinum-based chemotherapy.
Here, by contrast, sacituzumab tirumotecan is being tested directly against platinum-based chemotherapy in the second-line setting—earlier in the treatment course and against an active comparator.
If we look at other randomized ADC efforts in this population, such as patritumab deruxtecan, the HER3-targeting ADC presented at the American Society of Clinical Oncology annual meeting earlier this year, the results were much less convincing. That trial showed only a modest PFS improvement and no OS benefit.7
In contrast, this study provides a clear and substantial PFS advantage and a promising OS signal. Based on what we’ve seen so far, sacituzumab tirumotecan may ultimately prove more effective than datopotamab deruxtecan in this particular setting.
Luis Paz-Ares, MD, PhD:
Yes, I think so too. The magnitude of benefit here is quite striking. Now we simply need the global validation and mature OS data to determine whether this agent can move forward as a new standard. But scientifically and clinically, the trajectory looks very encouraging.